Breaking News: FDA Approves the MiniMed 670G System, World's First Hybrid Closed Loop System - The LOOP Blog
Por um escritor misterioso
Last updated 21 março 2025


Medtronic 670G is FDA approved
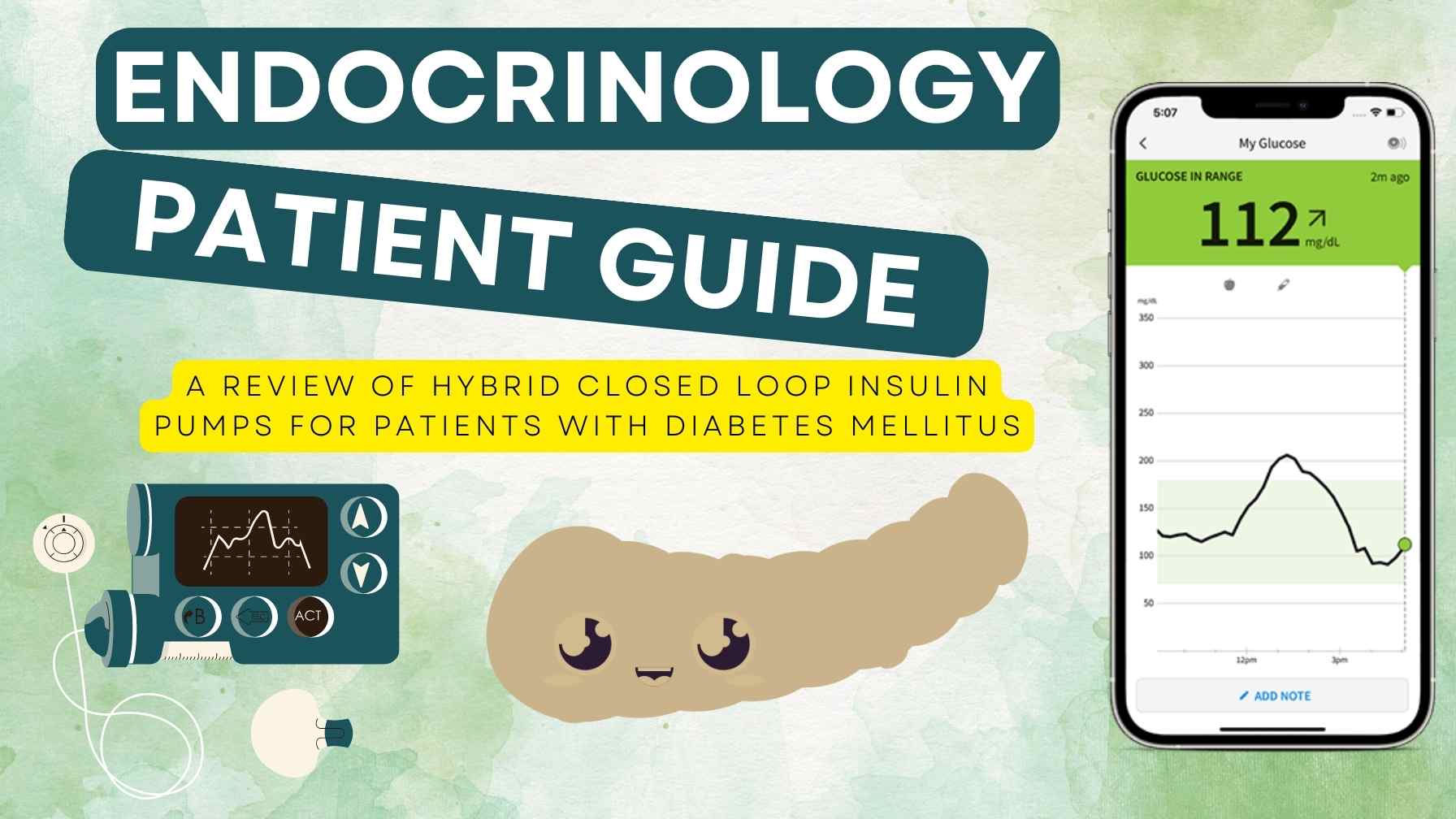
Best Insulin Pump : A Comprehensive Guide - My Endo Consult

FDA approves Medtronic's 'artificial pancreas' MinMed 670G hybrid closed loop system - MassDevice

hybrid closed loop Archives - The LOOP Blog
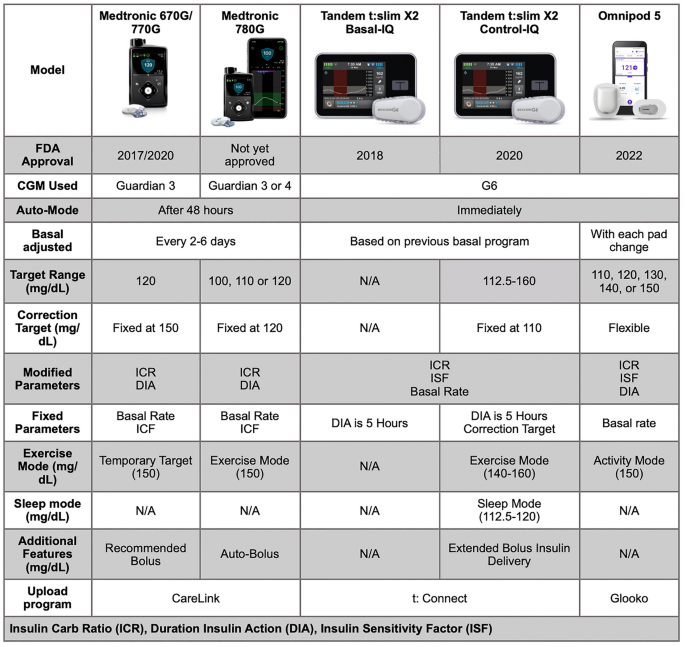
Emerging Diabetes Technologies: Continuous Glucose Monitors/Artificial Pancreases

The playing field is quickly - Diabetes Youth Families
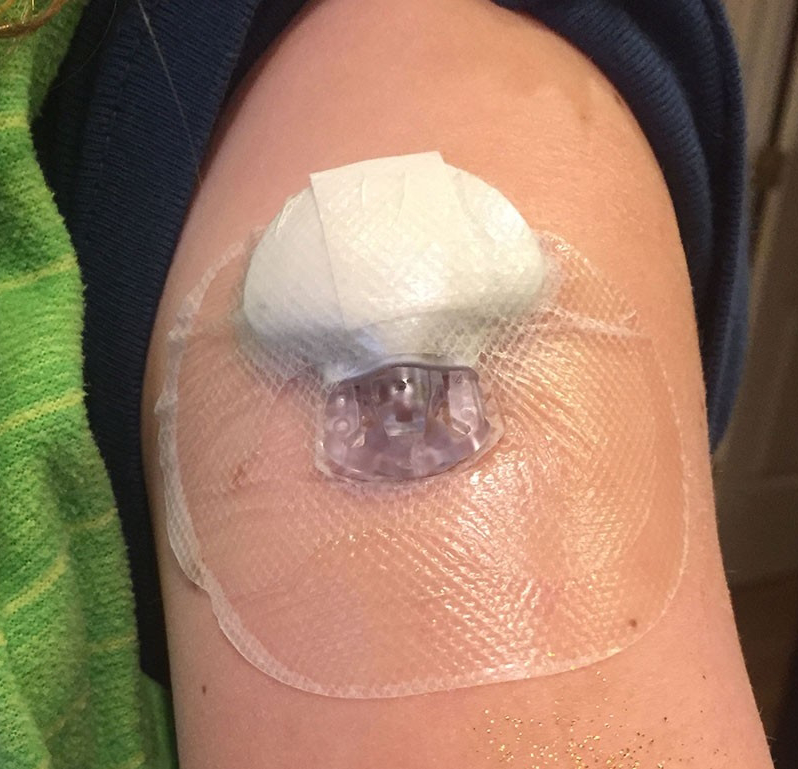
Hybrid closed-loop insulin delivery systems for Type 1 diabetes come of age

The MiniMed 780G - A deep dive into Medtronic's most advanced system - Diabetes Connections

Introducing the MiniMed 670G Hybrid Closed Loop System

The LOOP Blog Medtronic Diabetes Online Community
Recomendado para você
-
Mini Box Aguiar21 março 2025
-
maxPromotor – Apps on Google Play21 março 2025
-
 Eventos Aguiar - Consulte disponibilidade e preços21 março 2025
Eventos Aguiar - Consulte disponibilidade e preços21 março 2025 -
 Kit de Cartonagem Maleta de Costura Milly by Simone Aguiar – KitPat Cartonagem21 março 2025
Kit de Cartonagem Maleta de Costura Milly by Simone Aguiar – KitPat Cartonagem21 março 2025 -
 Polícia Militar do Amapá realiza Operação Saturação no bairro dos Congós – Silvio Sousa21 março 2025
Polícia Militar do Amapá realiza Operação Saturação no bairro dos Congós – Silvio Sousa21 março 2025 -
 Entenda o que é o Market Share e descubra as suas funcionalidades!21 março 2025
Entenda o que é o Market Share e descubra as suas funcionalidades!21 março 2025 -
 Bolsa em couro rosé Didê Mini21 março 2025
Bolsa em couro rosé Didê Mini21 março 2025 -
 SULA MAZUREGA: presenca de deus CHANTECLER 12 LP 33 RPM21 março 2025
SULA MAZUREGA: presenca de deus CHANTECLER 12 LP 33 RPM21 março 2025 -
 Tapi Go! - Clover Blog21 março 2025
Tapi Go! - Clover Blog21 março 2025 -
 Comerciais – Santo André – VILA AMÉRICA – KG IMÓVEIS21 março 2025
Comerciais – Santo André – VILA AMÉRICA – KG IMÓVEIS21 março 2025
você pode gostar
-
 Elomar - O Peão Na Amarração21 março 2025
Elomar - O Peão Na Amarração21 março 2025 -
 LIVE: JOGO DA DAMA NO HAGO + 💲GANHE DINHEIRO💲21 março 2025
LIVE: JOGO DA DAMA NO HAGO + 💲GANHE DINHEIRO💲21 março 2025 -
 Mejores webs y aplicaciones para jugar al ajedrez online21 março 2025
Mejores webs y aplicaciones para jugar al ajedrez online21 março 2025 -
 PlayStation Teases New PS5 Game From Days Gone Developer21 março 2025
PlayStation Teases New PS5 Game From Days Gone Developer21 março 2025 -
 Microsoft Xbox One S 500GB Console (White) - Pre-Owned21 março 2025
Microsoft Xbox One S 500GB Console (White) - Pre-Owned21 março 2025 -
 Jogos do Rei Leão em Jogos na Internet21 março 2025
Jogos do Rei Leão em Jogos na Internet21 março 2025 -
 𝐁𝐥𝐨𝐱𝐛𝐮𝐫𝐠 𝐌𝐮𝐬𝐢𝐜 𝐂𝐨𝐝𝐞𝐬 🎀✨21 março 2025
𝐁𝐥𝐨𝐱𝐛𝐮𝐫𝐠 𝐌𝐮𝐬𝐢𝐜 𝐂𝐨𝐝𝐞𝐬 🎀✨21 março 2025 -
 Forza Horizon: The Greatest Racing Game of All Time21 março 2025
Forza Horizon: The Greatest Racing Game of All Time21 março 2025 -
 Chuva suspende partida entre Corinthians e São José no Paulista Feminino; veja informações21 março 2025
Chuva suspende partida entre Corinthians e São José no Paulista Feminino; veja informações21 março 2025 -
 NetBenefits Login Page - Fidelity21 março 2025
NetBenefits Login Page - Fidelity21 março 2025
