FDA won't comment on status of Emergency Use Authorizations for two antibody treatments
Por um escritor misterioso
Last updated 30 março 2025

The US Food and Drug Administration told CNN Thursday morning that the agency doesn’t have any comments on the applications for Emergency Use Authorizations for Eli Lilly and Regeneron antibody treatments.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.

Regulatory tracker: EMA backs Vertex's gene-editing therapy

Moderna asks the F.D.A. to authorize its vaccine for children

F.D.A. Allows Expanded Use of Convalescent Plasma to Treat
Emergency Use Authorization

Federal Register :: Authorizations of Emergency Use of Certain

Emergency Use Authorization

FDA announces emergency authorization of plasma treatment for COVID-19
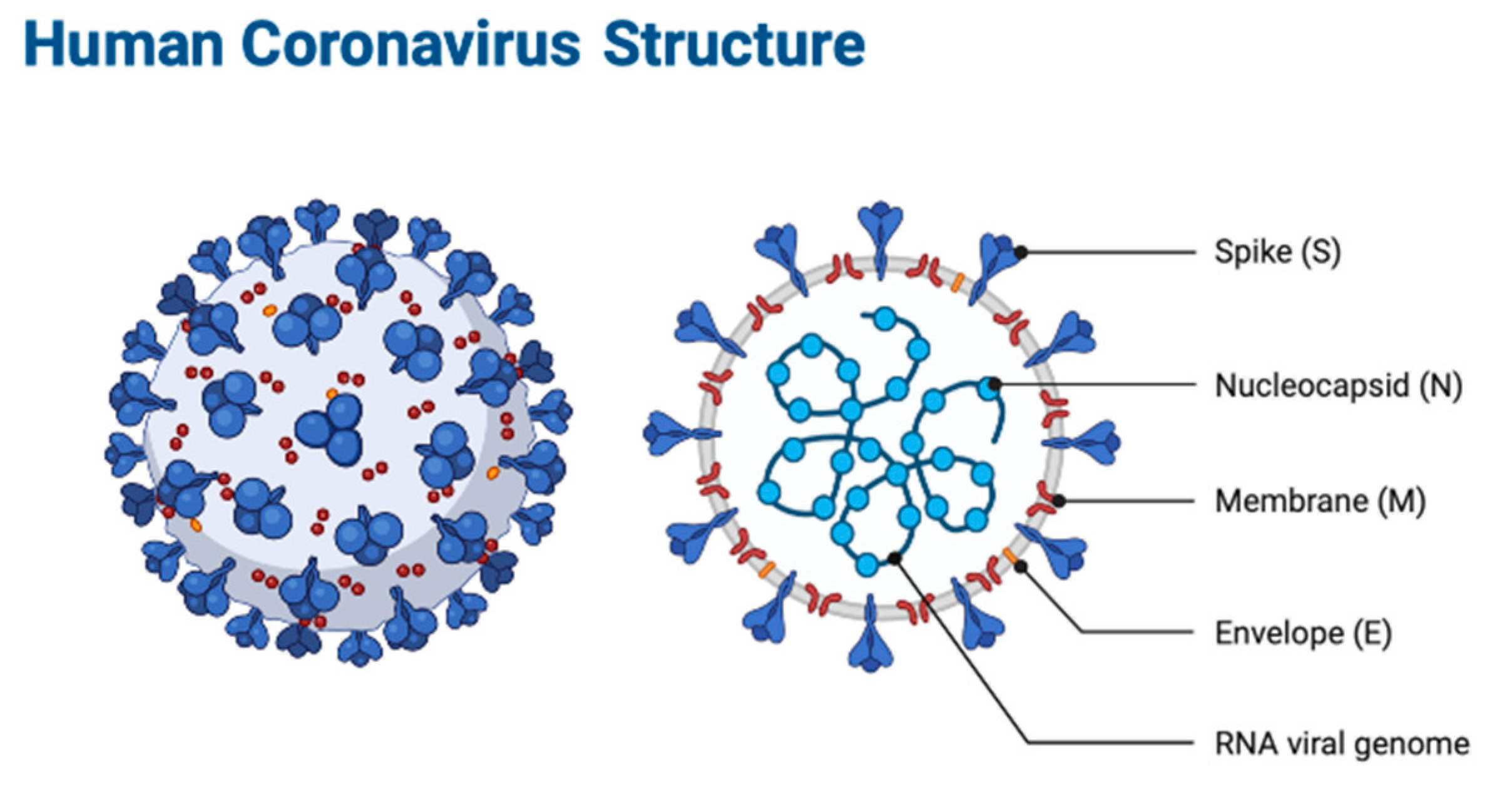
IJMS, Free Full-Text

Covid-19: F.D.A. Panel Gives Green Light to Johnson & Johnson's

FDA ends for now use of two monoclonal antibodies, spurring a halt

FDA Revokes Emergency Use Authorization for Monoclonal

Federal Register :: Authorizations of Emergency Use of Certain

Coronavirus Drug and Treatment Tracker - The New York Times
Food and Drug Administration Open Meeting on Moderna COVID-19
Recomendado para você
-
 Isaac Azar E Karol Bang (1)30 março 2025
Isaac Azar E Karol Bang (1)30 março 2025 -
 Agenda: Vips no Uruguai30 março 2025
Agenda: Vips no Uruguai30 março 2025 -
 Mahmoud Al-Hawary MD Anderson Cancer Center30 março 2025
Mahmoud Al-Hawary MD Anderson Cancer Center30 março 2025 -
Surgical Endoscopy30 março 2025
-
 Dr. Marwan Azar, MD, New Haven, CT, Infectious Disease Specialist30 março 2025
Dr. Marwan Azar, MD, New Haven, CT, Infectious Disease Specialist30 março 2025 -
Josh Azar30 março 2025
-
Israeli Post-doctoral Fellows30 março 2025
-
 Safety Announcements30 março 2025
Safety Announcements30 março 2025 -
 The Bible: An Islamic Perspective - Abraham: Jay R. Crook: 9781567447460: : Books30 março 2025
The Bible: An Islamic Perspective - Abraham: Jay R. Crook: 9781567447460: : Books30 março 2025 -
 Pediatric Specialty Care, Jacksonville30 março 2025
Pediatric Specialty Care, Jacksonville30 março 2025
você pode gostar
-
Local Time - Automatic Time Zone Converter30 março 2025
-
 Chewmeter Game Valorant Prime Axe Model Action Figures Game Toys30 março 2025
Chewmeter Game Valorant Prime Axe Model Action Figures Game Toys30 março 2025 -
 Palkia - Pokémon Wiki - Neoseeker30 março 2025
Palkia - Pokémon Wiki - Neoseeker30 março 2025 -
Ele aproveitou a oportunidade #animeslegais #animes #cenasdeanimes30 março 2025
-
 Como chegar até Complexo Viário Heróis de 1932 (Cebolão) em Vila Leopoldina de Ônibus, Trem ou Metrô?30 março 2025
Como chegar até Complexo Viário Heróis de 1932 (Cebolão) em Vila Leopoldina de Ônibus, Trem ou Metrô?30 março 2025 -
 n/a Beach Wear Clothes Men Shirt Set Sea Side Vocation Clothing Loose 2 Piece Set Outfits (Color : A, Size : XXXL code) : : Fashion30 março 2025
n/a Beach Wear Clothes Men Shirt Set Sea Side Vocation Clothing Loose 2 Piece Set Outfits (Color : A, Size : XXXL code) : : Fashion30 março 2025 -
 Pou 2 Memes Amino • Español Amino30 março 2025
Pou 2 Memes Amino • Español Amino30 março 2025 -
 Loading Screen, the last of us ii, tlou 2, HD wallpaper30 março 2025
Loading Screen, the last of us ii, tlou 2, HD wallpaper30 março 2025 -
 Bandeira do Reino Unido Londres 2012 imagem vetorial de30 março 2025
Bandeira do Reino Unido Londres 2012 imagem vetorial de30 março 2025 -
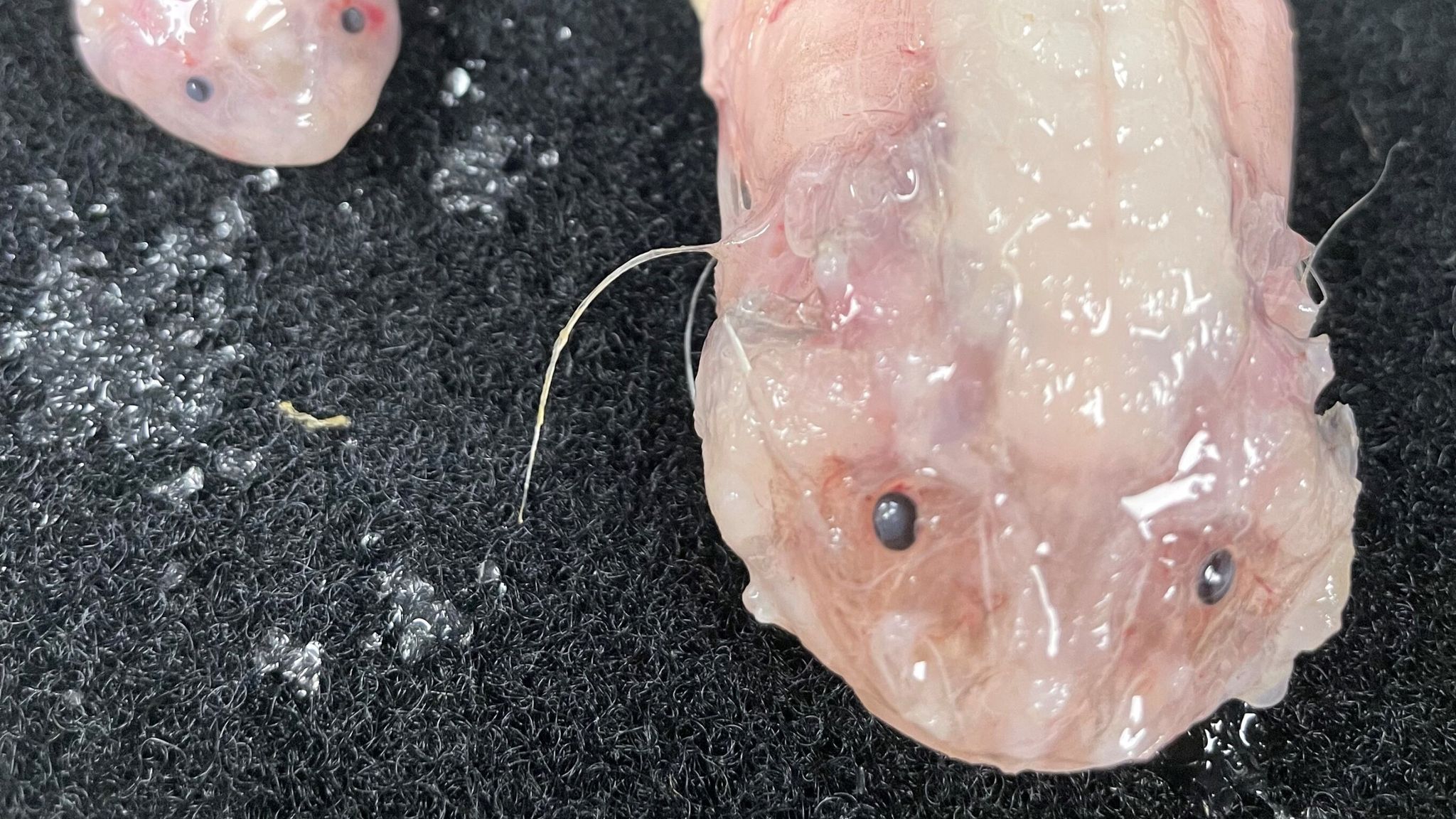 Deepest fish ever recorded revealed by scientists30 março 2025
Deepest fish ever recorded revealed by scientists30 março 2025

