New approach methodologies in human regulatory toxicology – Not if, but how and when! - ScienceDirect
Por um escritor misterioso
Last updated 30 março 2025

PDF) Mechanistic integration of exposure and effects: Advances to apply systems toxicology in support of regulatory decision making

From Basic Research in Toxicology to a Career in Regulatory Toxicology: An Industry Perspective

Testing the feasibility of a new way of toxicity testing and reduction

Regulatory Science – An Underappreciated Component of Translational Research: Trends in Pharmacological Sciences

Innovation in regulatory approaches for endocrine disrupting chemicals: The journey to risk assessment modernization in Canada - ScienceDirect
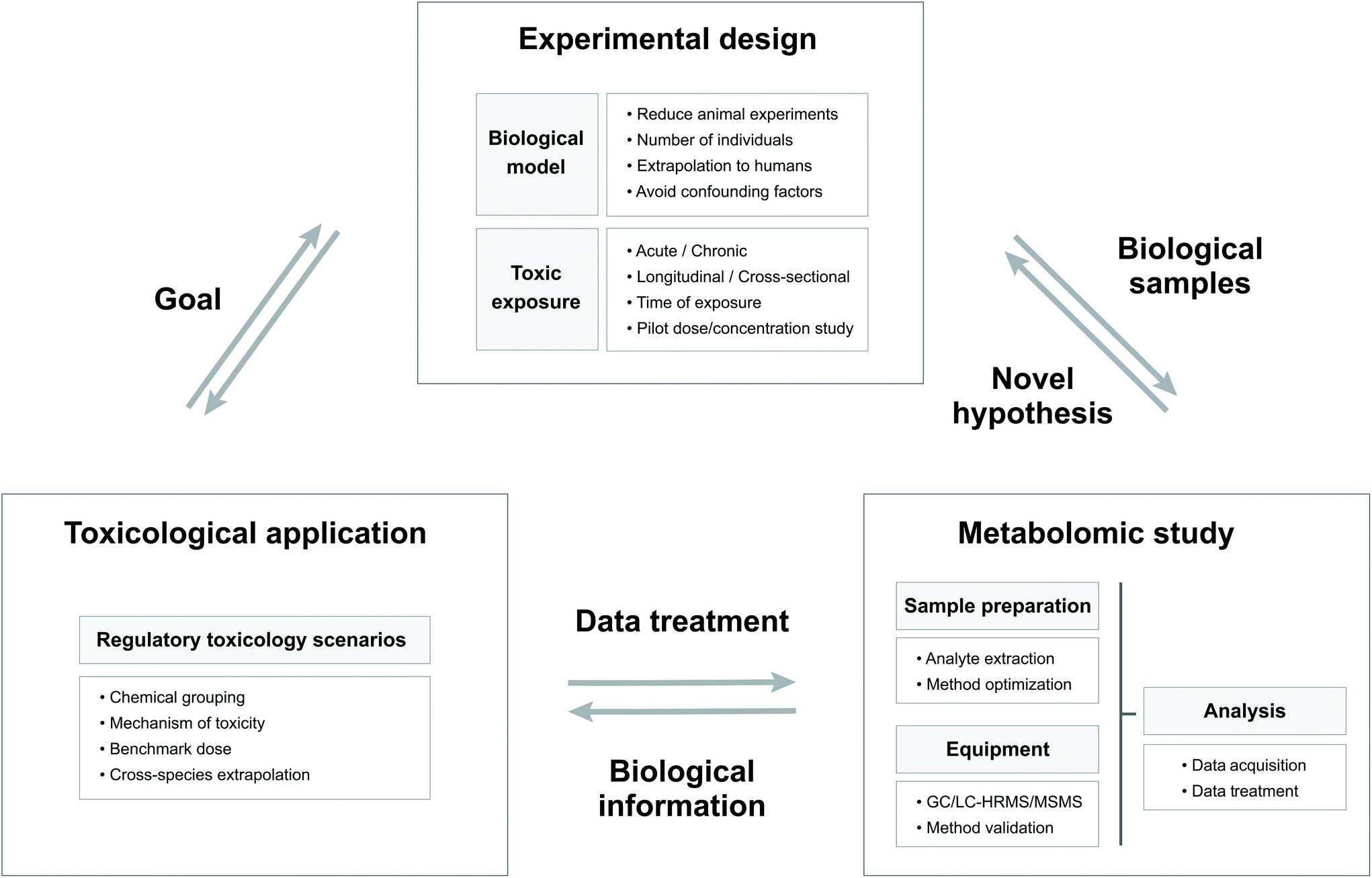
Approaches in metabolomics for regulatory toxicology applications - Analyst (RSC Publishing) DOI:10.1039/D0AN02212H
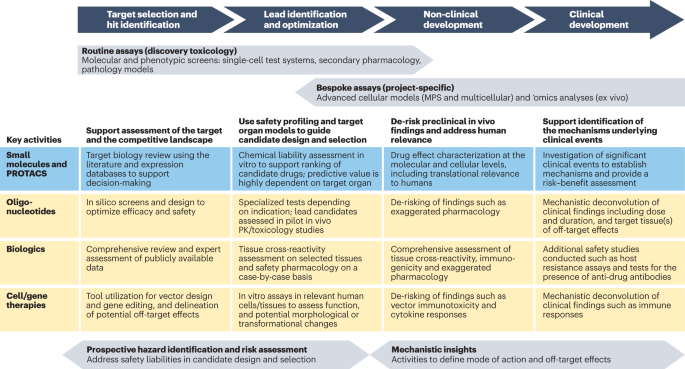
The evolving role of investigative toxicology in the pharmaceutical industry

Toxicity testing: creating a revolution based on new technologies: Trends in Biotechnology

New approach methodologies in human regulatory toxicology – Not if, but how and when! - ScienceDirect

Regulatory Science Approach in Pharmaceutical Development of Follow-on Versions of Non-Biological Complex Drug Products - Journal of Pharmaceutical Sciences

Envisioning an international validation process for New Approach Methodologies in chemical hazard and risk assessment - ScienceDirect

An evaluation framework for new approach methodologies (NAMs) for human health safety assessment - ScienceDirect

New Approach Methodologies (NAMs) for safety testing of complex food matrices: A review of status, considerations, and regulatory adoption - ScienceDirect

Systems toxicology to advance human and environmental hazard assessment: A roadmap for advanced materials - ScienceDirect

A Call For Action On The Development and Implementation o - 2021 - Regulatory To, PDF, Toxicology
Recomendado para você
-
 Press Archive - Stanford Law School30 março 2025
Press Archive - Stanford Law School30 março 2025 -
 Central America & the Caribbean Archives - The Dialogue30 março 2025
Central America & the Caribbean Archives - The Dialogue30 março 2025 -
Kelly Godoy (@Kellygodoyjor) / X30 março 2025
-
Kelly Godoy30 março 2025
-
 Kelly Godoy (godoy0813) - Profile30 março 2025
Kelly Godoy (godoy0813) - Profile30 março 2025 -
Dancing with the Stars - Wikipedia30 março 2025
-
 About: Mary Ellen Lepionka - Indigenous History of Essex County, Massachusetts30 março 2025
About: Mary Ellen Lepionka - Indigenous History of Essex County, Massachusetts30 março 2025 -
kelly godoy (@GodoySexy) / X30 março 2025
-
 Pin on Doggy30 março 2025
Pin on Doggy30 março 2025 -
 Brazilian Volleyball Biography Introduction: Gilberto Godoy Filho, Hélia Souza, Ricardo Santos, Emanuel Rego, Larissa França, Gustavo Endres : LLC, Books: : Libros30 março 2025
Brazilian Volleyball Biography Introduction: Gilberto Godoy Filho, Hélia Souza, Ricardo Santos, Emanuel Rego, Larissa França, Gustavo Endres : LLC, Books: : Libros30 março 2025
você pode gostar
-
 SCP 008 Vs Virus-T (Death battle) by DemonFamily on DeviantArt30 março 2025
SCP 008 Vs Virus-T (Death battle) by DemonFamily on DeviantArt30 março 2025 -
/i.s3.glbimg.com/v1/AUTH_08fbf48bc0524877943fe86e43087e7a/internal_photos/bs/2021/q/4/Voa9upQKGVbBTzkDTOLA/2016-04-04-google-play-icons-blogpost.png) Play Store e outros apps do Google ganham novo ícone no Android30 março 2025
Play Store e outros apps do Google ganham novo ícone no Android30 março 2025 -
 Kit Conjunto Mochila Escolar Infantil Luccas Neto Rodinhas30 março 2025
Kit Conjunto Mochila Escolar Infantil Luccas Neto Rodinhas30 março 2025 -
 Here's what playing Minecraft inside Minecraft looks like30 março 2025
Here's what playing Minecraft inside Minecraft looks like30 março 2025 -
 fofa pequeno Mago dia das Bruxas clipart ilustração ai generativo30 março 2025
fofa pequeno Mago dia das Bruxas clipart ilustração ai generativo30 março 2025 -
 Jogo do Bicho Online Melhores Sites do Brasil 2022 🇧🇷30 março 2025
Jogo do Bicho Online Melhores Sites do Brasil 2022 🇧🇷30 março 2025 -
 ps5 pro - Best Buy30 março 2025
ps5 pro - Best Buy30 março 2025 -
 Barbie para colorir Desenhos para colorir barbie, Colorir barbie, Páginas para colorir para adultos30 março 2025
Barbie para colorir Desenhos para colorir barbie, Colorir barbie, Páginas para colorir para adultos30 março 2025 -
 Georgia Judge Christian Coomer Moves to Dismiss 33 of 36 Ethics Charges Against Him30 março 2025
Georgia Judge Christian Coomer Moves to Dismiss 33 of 36 Ethics Charges Against Him30 março 2025 -
 Graphics Quality Changer Plugin - Community Resources - Developer Forum30 março 2025
Graphics Quality Changer Plugin - Community Resources - Developer Forum30 março 2025


