hydrogen orbital wavefunction
Por um escritor misterioso
Last updated 06 janeiro 2025
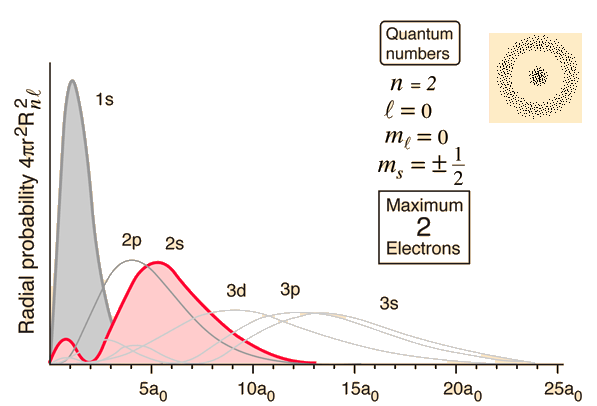
Hydrogen Radial Probabilities

How to determine the Ground State wavefunction of the Hydrogen Atom (n=1, l=0, m=0)

11.10: The Schrödinger Wave Equation for the Hydrogen Atom - Chemistry LibreTexts

quantum mechanics - How do we decide whether an electron orbital has a non-zero or zero probability of lying inside the nucleus of an hydrogen atom? - Physics Stack Exchange
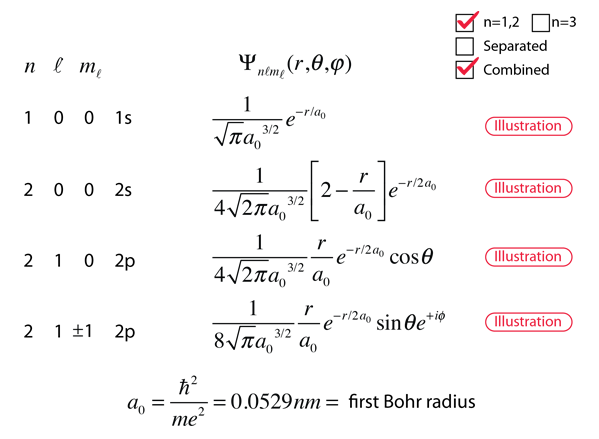
Hydrogen Wavefunctions
The wave function of hydrogen atom with its electron in the 2p state varies with direction as well as distance from the nucleus. What is the probability of a 2p electron, for

Hydrogen-like atom - Wikipedia
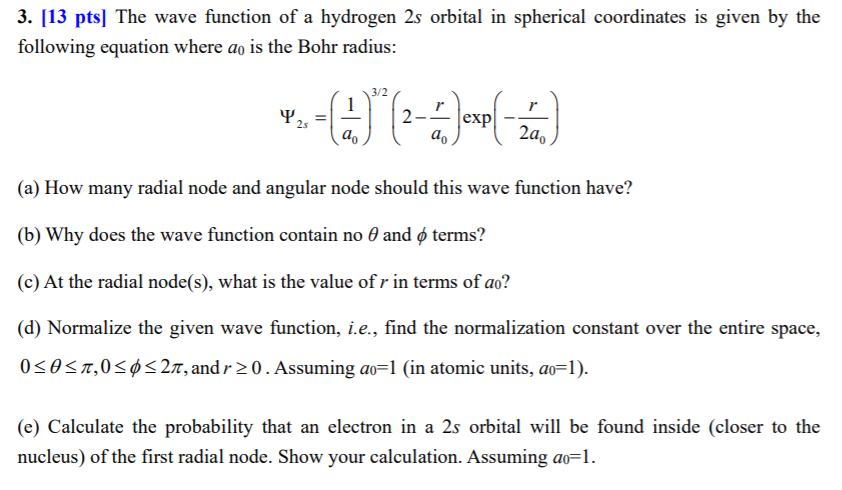
Solved 3. [13 pts] The wave function of a hydrogen 2s

For 1s orbital of Hydrogen atom radial wave function is given as: R(r)=1/√(π)(1/a_0)^3 / 2 e^-r /

The Hydrogen Wave Function, Imaged, Science
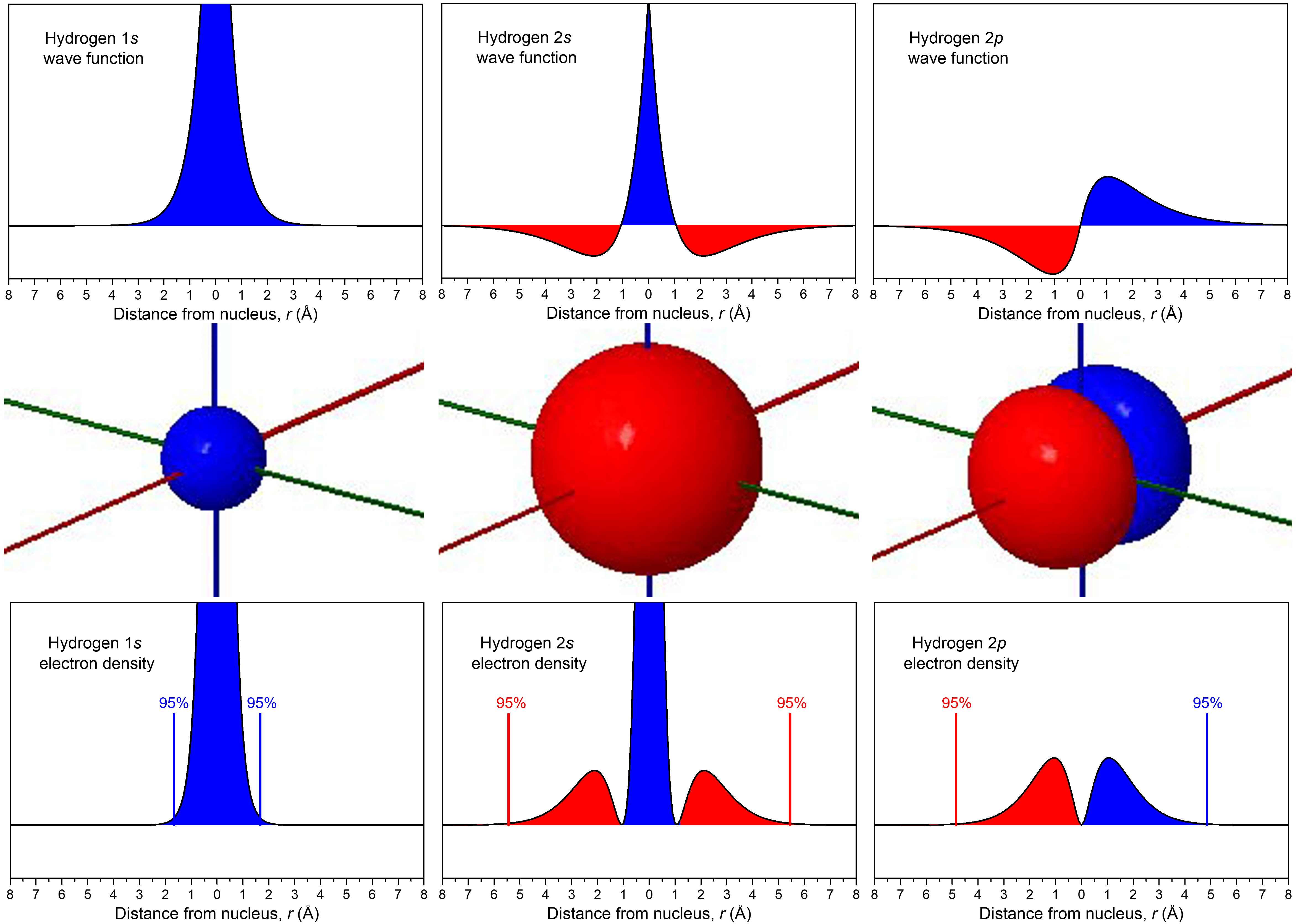
D3.1 Atomic Orbitals and Quantum Numbers – Chemistry 109 Fall 2021

Radial and Angular Parts of Atomic Orbitals - Chemistry LibreTexts
Recomendado para você
-
Gacha Life Club Wallpaper Cute - Apps on Google Play06 janeiro 2025
-
 GUERLAIN Mad Eyes Felt-tip Precision Eyeliner 0.6ml06 janeiro 2025
GUERLAIN Mad Eyes Felt-tip Precision Eyeliner 0.6ml06 janeiro 2025 -
 Pin on Salvamentos rápidos06 janeiro 2025
Pin on Salvamentos rápidos06 janeiro 2025 -
Italo Rodrigues - Bom aqui está como eu disse, terceira06 janeiro 2025
-
 Free Vector Hand drawn angry mouth cartoon illustration06 janeiro 2025
Free Vector Hand drawn angry mouth cartoon illustration06 janeiro 2025 -
 Continuous line water drop art droplet icon rain outline sketch doodle drawing. One line linear blood sea water drop drawn tear eco donation abstract medical simple logo isolated. Vector Illustration 30308189 Vector06 janeiro 2025
Continuous line water drop art droplet icon rain outline sketch doodle drawing. One line linear blood sea water drop drawn tear eco donation abstract medical simple logo isolated. Vector Illustration 30308189 Vector06 janeiro 2025 -
![✨How To Draw GACHA Life Mouths✨[ How I draw mouths GACHA life ] Gacha Life Tutorial! [ GIVE CREDIT ]](https://i.ytimg.com/vi/cIrPA9z7d0U/maxresdefault.jpg) ✨How To Draw GACHA Life Mouths✨[ How I draw mouths GACHA life ] Gacha Life Tutorial! [ GIVE CREDIT ]06 janeiro 2025
✨How To Draw GACHA Life Mouths✨[ How I draw mouths GACHA life ] Gacha Life Tutorial! [ GIVE CREDIT ]06 janeiro 2025 -
 Amusement Authority: Verbolten 3D CAD Model06 janeiro 2025
Amusement Authority: Verbolten 3D CAD Model06 janeiro 2025 -
 Choose When You'll Be MaMa's Boy In Gacha Life To Find Out Something Shocking About Your Personality. - - Free Photo Effects & Trending Quizzes06 janeiro 2025
Choose When You'll Be MaMa's Boy In Gacha Life To Find Out Something Shocking About Your Personality. - - Free Photo Effects & Trending Quizzes06 janeiro 2025 -
 📱\ Dicas de edição #4: Como fazer uma boca realista ~How to make realistic mouth~ ·Gacha Club· /📱\06 janeiro 2025
📱\ Dicas de edição #4: Como fazer uma boca realista ~How to make realistic mouth~ ·Gacha Club· /📱\06 janeiro 2025
você pode gostar
-
 Samsung Galaxy S21 Ultra - SamMobile06 janeiro 2025
Samsung Galaxy S21 Ultra - SamMobile06 janeiro 2025 -
 He's Money: The Highest Paid Players in NBA History06 janeiro 2025
He's Money: The Highest Paid Players in NBA History06 janeiro 2025 -
 JoJo's Bizarre Adventure: Eyes of Heaven Review - PlayLab! Magazine06 janeiro 2025
JoJo's Bizarre Adventure: Eyes of Heaven Review - PlayLab! Magazine06 janeiro 2025 -
Record of Ragnarok (dublado)EP 1 temp 1°, By Animes jb06 janeiro 2025
-
 JoJo's Bizarre Adventure: Stone Ocean – 01, 02, 03 – Random Curiosity06 janeiro 2025
JoJo's Bizarre Adventure: Stone Ocean – 01, 02, 03 – Random Curiosity06 janeiro 2025 -
![BEST MOUSE ONLY GAMES FOR PC [2022 UPDATE!]](https://i.ytimg.com/vi/_RFdEBUxc5U/hqdefault.jpg) BEST MOUSE ONLY GAMES FOR PC [2022 UPDATE!]06 janeiro 2025
BEST MOUSE ONLY GAMES FOR PC [2022 UPDATE!]06 janeiro 2025 -
 GRID™ Autosport Custom Edition (iOS & Android) - First Look06 janeiro 2025
GRID™ Autosport Custom Edition (iOS & Android) - First Look06 janeiro 2025 -
 T2:E3 - Episódio 3 - Gold Diggers: Luxúria e Poder online no Globoplay06 janeiro 2025
T2:E3 - Episódio 3 - Gold Diggers: Luxúria e Poder online no Globoplay06 janeiro 2025 -
 Veja imagens do Kakashi personagem do anime Naruto e aprenda a gostar mais do anime faça as fotos de papel de parede - …06 janeiro 2025
Veja imagens do Kakashi personagem do anime Naruto e aprenda a gostar mais do anime faça as fotos de papel de parede - …06 janeiro 2025 -
 como reembolsar um jogo na steam|Pesquisa do TikTok06 janeiro 2025
como reembolsar um jogo na steam|Pesquisa do TikTok06 janeiro 2025

