FDA allows Houston cancer doctor to resume drug trial
Por um escritor misterioso
Last updated 14 fevereiro 2025

Federal regulators have lifted a partial hold on a clinical trial performed by Stanislaw

Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial - The Lancet Oncology

Katy Rezvani MD Anderson Cancer Center

Gene Editing for Cancer Is Coming of Age, Article

At the Bedside: Pancreatic cancer patient 'back on track' after UTHealth Houston clinical trial therapy shrinks tumor - UTHealth Houston

Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study - The Lancet Oncology

Funda Meric-Bernstam MD Anderson Cancer Center

UTHealth Houston

Doctor claims to cure pediatric cancer, critics skeptical
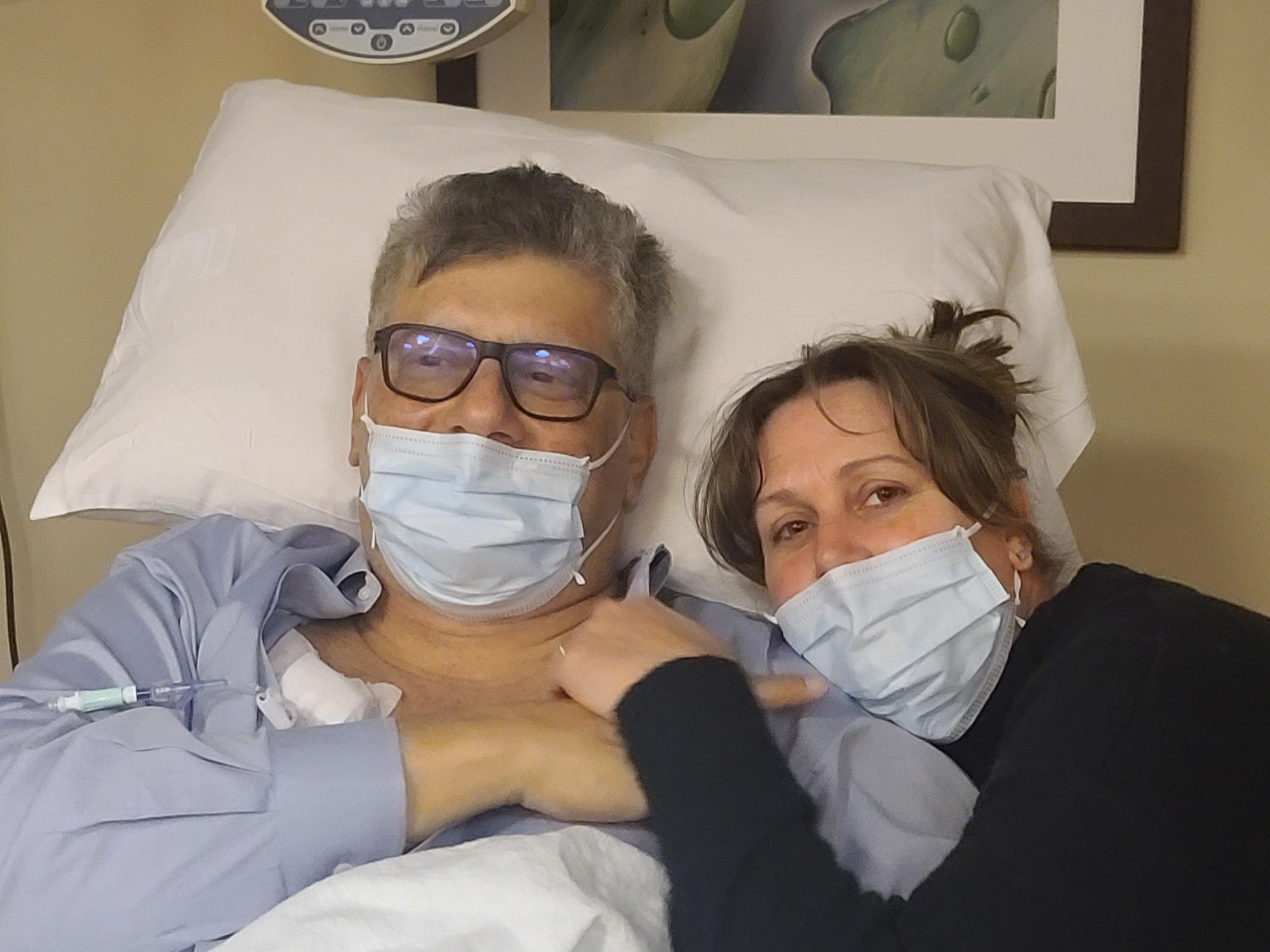
At the Bedside: Pancreatic cancer patient 'back on track' after UTHealth Houston clinical trial therapy shrinks tumor - UTHealth Houston

FDA panel: Benefit of 'highly anticipated' lung cancer drug can't be interpreted reliably
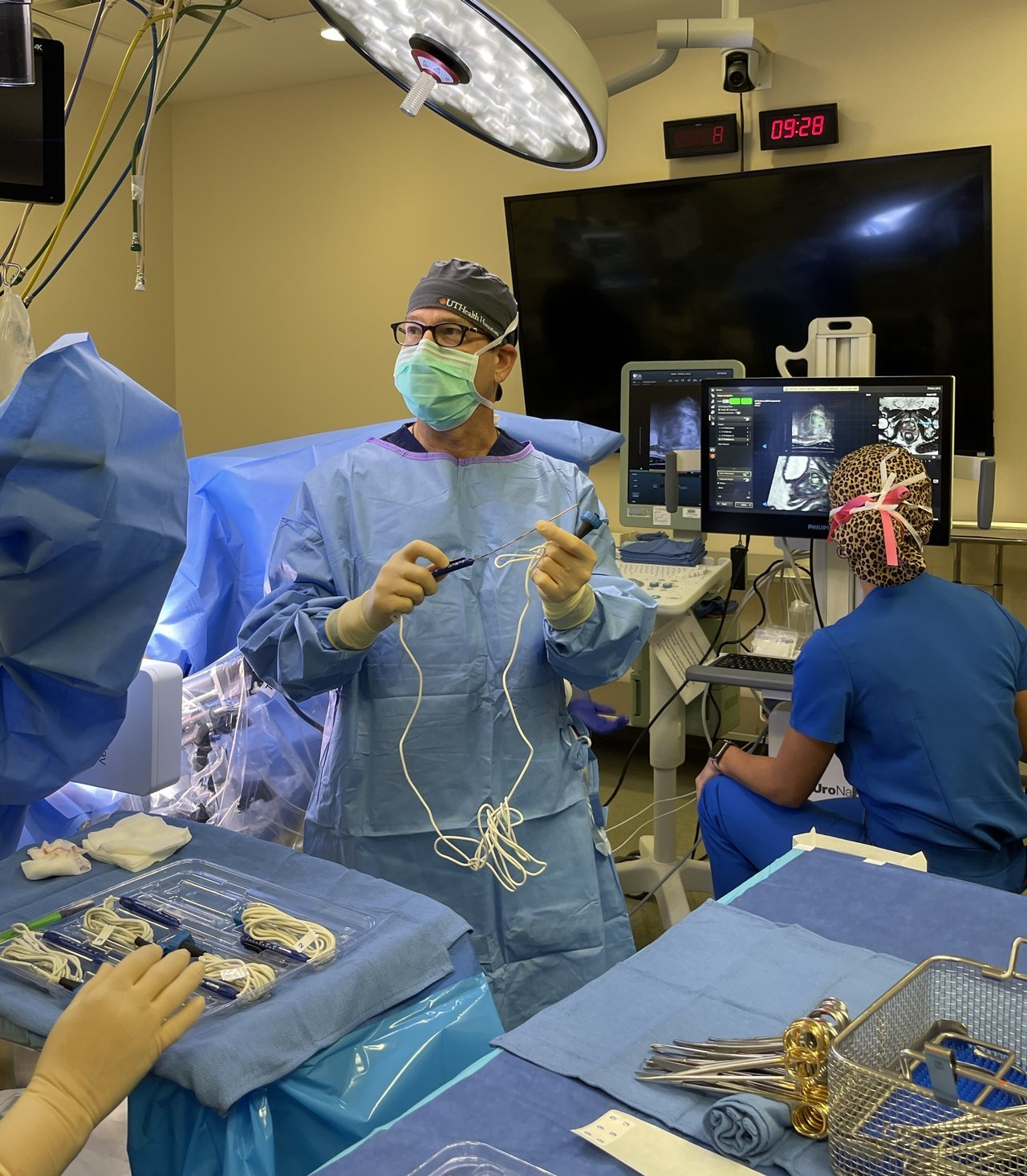
At the Bedside: New focal therapies at UTHealth Houston make prostate cancer treatment easier on patients - UTHealth News - UTHealth Houston

FibroGen limited access to Duchenne muscular dystrophy drug after failed clinical trial. But mom says it works for her son. - San Francisco Business Times

Ashish M. Kamat MD Anderson Cancer Center

FDA agrees to let patients get controversial drug
Novartis challenges Pfizer with strong breast cancer drug data
Recomendado para você
-
 What is Dr Now's net worth?14 fevereiro 2025
What is Dr Now's net worth?14 fevereiro 2025 -
 My 600-Lb Life': Shannon Lowery Struggling With Move to Houston for Surgery With Dr. Now14 fevereiro 2025
My 600-Lb Life': Shannon Lowery Struggling With Move to Houston for Surgery With Dr. Now14 fevereiro 2025 -
 Dr now houston tx office visit from fan|TikTok Search14 fevereiro 2025
Dr now houston tx office visit from fan|TikTok Search14 fevereiro 2025 -
Houston Methodist Willowbrook now offers minimally invasive TAVR procedure, providing faster recovery for patients with heart valve disease14 fevereiro 2025
-
 Dr. Now MD on Instagram: “How y'all doing? #motivationmonday ShopDrNow.com fridge magnets! #drnowmd #shopdrnow #weight…14 fevereiro 2025
Dr. Now MD on Instagram: “How y'all doing? #motivationmonday ShopDrNow.com fridge magnets! #drnowmd #shopdrnow #weight…14 fevereiro 2025 -
 Dr. Caron Houston Makes House Calls - Inside Sacramento14 fevereiro 2025
Dr. Caron Houston Makes House Calls - Inside Sacramento14 fevereiro 2025 -
 My signed copy of Dr.Now's book I got in the mail today! It's a little dented from USPS but I'm still excited 🥲 : r/My600lbLife14 fevereiro 2025
My signed copy of Dr.Now's book I got in the mail today! It's a little dented from USPS but I'm still excited 🥲 : r/My600lbLife14 fevereiro 2025 -
 7520 Hornwood Dr Apt 607, Houston, TX 7703614 fevereiro 2025
7520 Hornwood Dr Apt 607, Houston, TX 7703614 fevereiro 2025 -
 Spring Branch Community Health Center on X: We're proud to welcome Dr. Ashraf Elmeery, our newest Pediatric Provider to West Houston Community Health Center. Dr. Elmeery is now accepting new patients. Learn14 fevereiro 2025
Spring Branch Community Health Center on X: We're proud to welcome Dr. Ashraf Elmeery, our newest Pediatric Provider to West Houston Community Health Center. Dr. Elmeery is now accepting new patients. Learn14 fevereiro 2025 -
 The giver – Dentistry News14 fevereiro 2025
The giver – Dentistry News14 fevereiro 2025
você pode gostar
-
 Shazam!' sequel tops North America box office, but results was considered 'disappointing14 fevereiro 2025
Shazam!' sequel tops North America box office, but results was considered 'disappointing14 fevereiro 2025 -
 The Worst ReAction Figures Wave 3C - X-2 (Monster Glow) SDCC 22 – Super714 fevereiro 2025
The Worst ReAction Figures Wave 3C - X-2 (Monster Glow) SDCC 22 – Super714 fevereiro 2025 -
 13 perguntas com pegadinhas para testar sua concentração - Listas14 fevereiro 2025
13 perguntas com pegadinhas para testar sua concentração - Listas14 fevereiro 2025 -
 Kaguya-sama: Love Is War, Vol. 21 Manga Super Anime Store14 fevereiro 2025
Kaguya-sama: Love Is War, Vol. 21 Manga Super Anime Store14 fevereiro 2025 -
 Black and white mess on Roblox Devforum - Forum Bugs - Developer14 fevereiro 2025
Black and white mess on Roblox Devforum - Forum Bugs - Developer14 fevereiro 2025 -
 Roblox Avatar Roblox history, Roblox, Avatar14 fevereiro 2025
Roblox Avatar Roblox history, Roblox, Avatar14 fevereiro 2025 -
 Camisa E Gravata Vetores, Ilustrações e Cliparts para Projetos14 fevereiro 2025
Camisa E Gravata Vetores, Ilustrações e Cliparts para Projetos14 fevereiro 2025 -
 Easter Egg clipart. Free download transparent .PNG14 fevereiro 2025
Easter Egg clipart. Free download transparent .PNG14 fevereiro 2025 -
 Ever After High First Chapter Briar Beauty Doll : Toys & Games14 fevereiro 2025
Ever After High First Chapter Briar Beauty Doll : Toys & Games14 fevereiro 2025 -
 ⭐️ POCOYO em PORTUGUÊS do BRASIL - Brilha brilha ⭐️14 fevereiro 2025
⭐️ POCOYO em PORTUGUÊS do BRASIL - Brilha brilha ⭐️14 fevereiro 2025
