What Does the IRB Review?, Research
Por um escritor misterioso
Last updated 21 março 2025

Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.
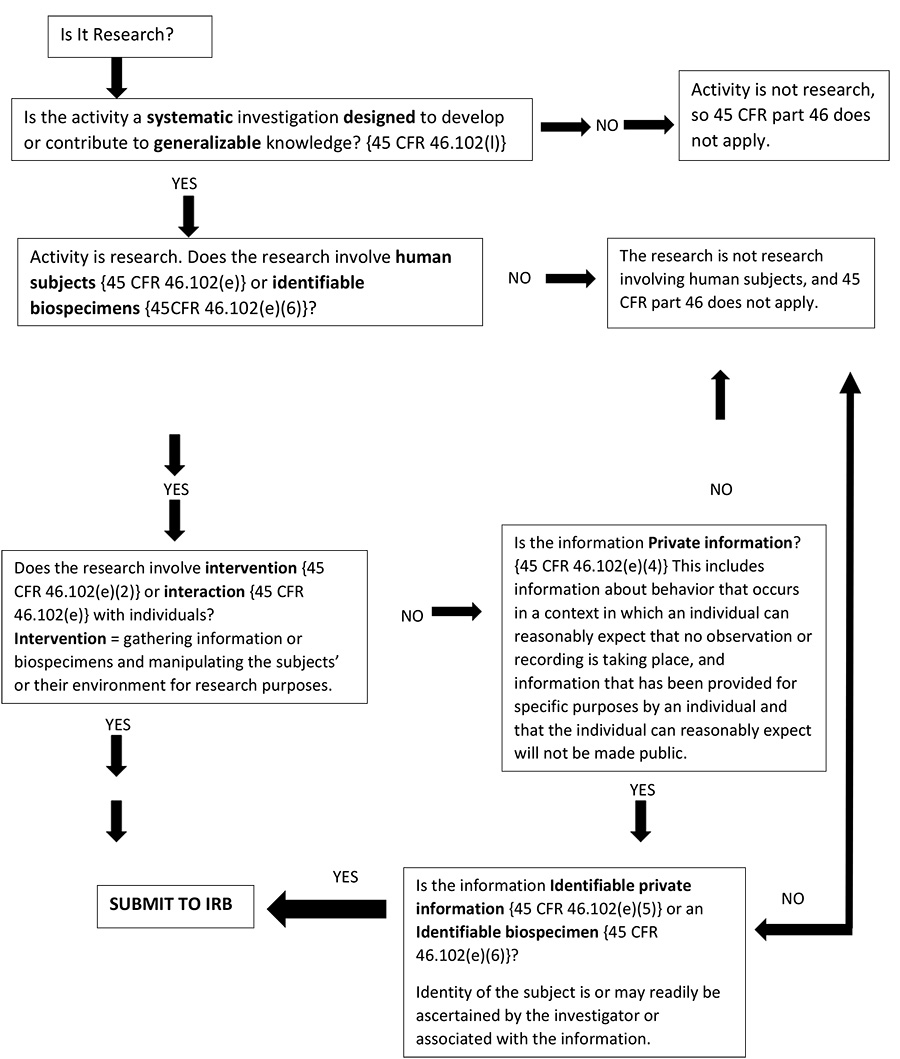
Does Your Research Project Require IRB Review?

What is an IRB?

IRB reliance: An informatics approach - ScienceDirect
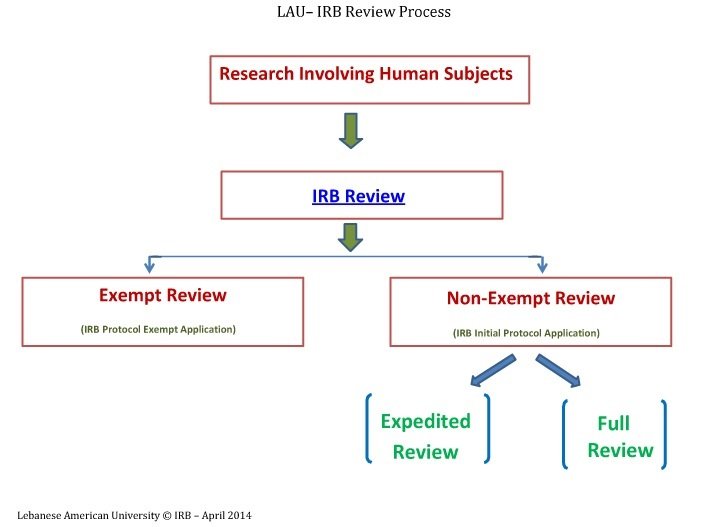
IRB Types of Review, Ethical Compliance, Graduate Studies and Research

Activities Requiring IRB Review - Office of Research Support and Compliance
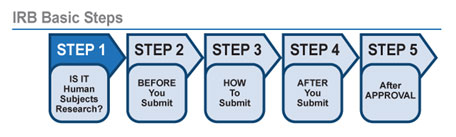
HUMAN SUBJECTS For Researchers U.S. DOE Office of Science (SC)

Levels of IRB Review

Requirements for Institutional Review Board (IRB) Review and HIPAA Waiver Documentation for RIF DUA Request Submissions
New Common Rule, 2019, IRB Blog, Institutional Review Board
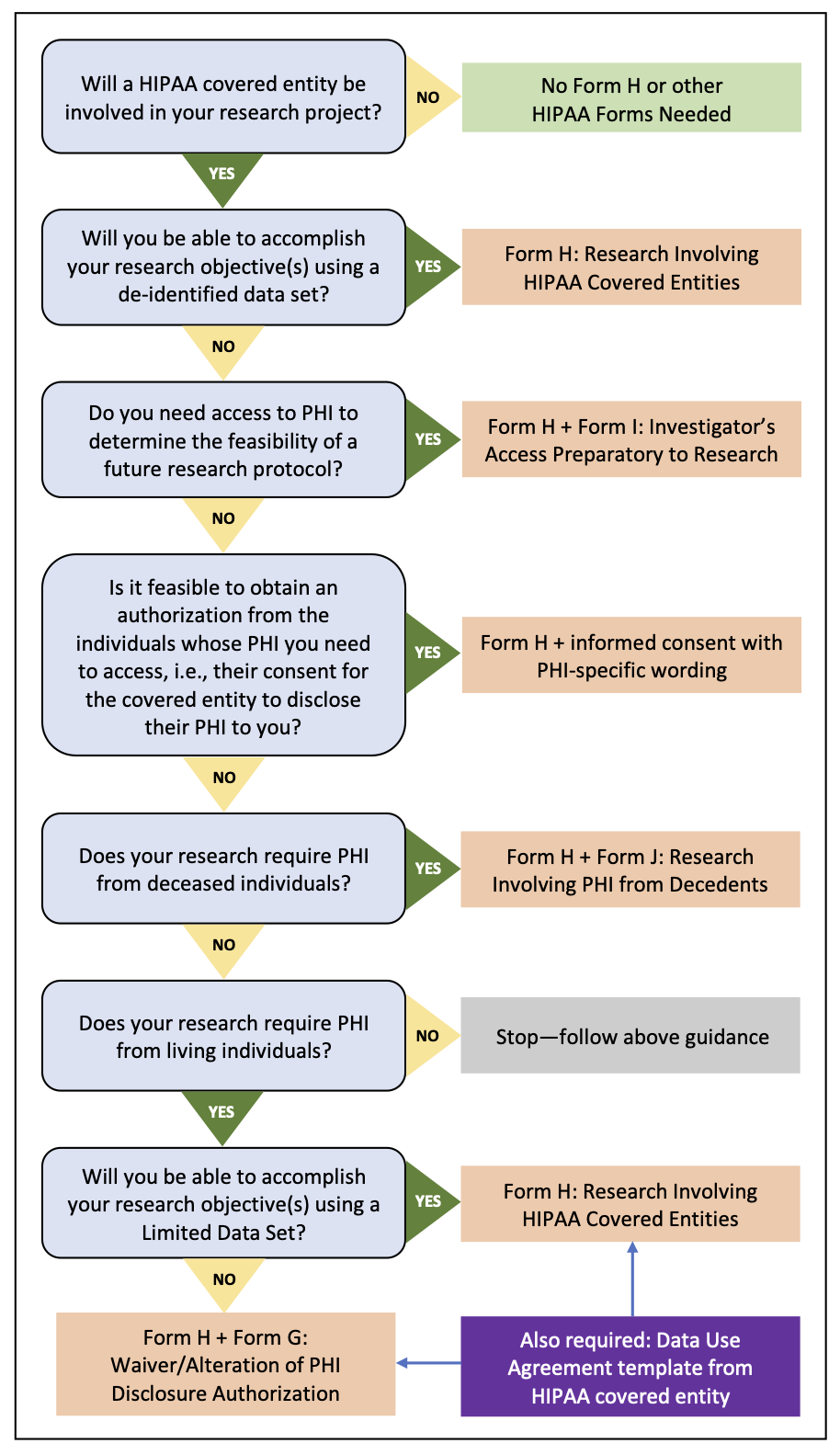
IRB Application and Review Process for Research Involving PHI

Frequently Asked Questions (FAQ)
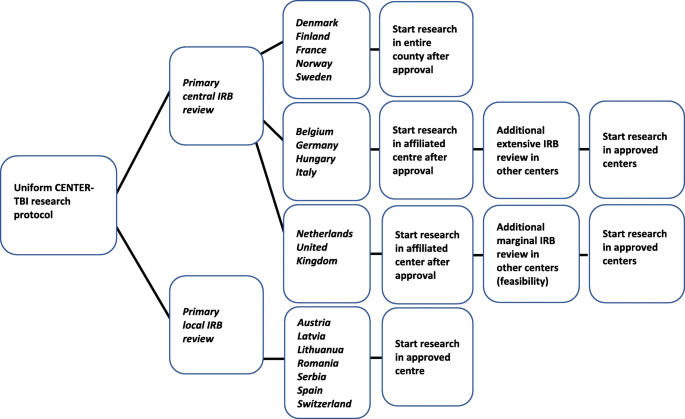
How do 66 European institutional review boards approve one protocol for an international prospective observational study on traumatic brain injury? Experiences from the CENTER-TBI study, BMC Medical Ethics
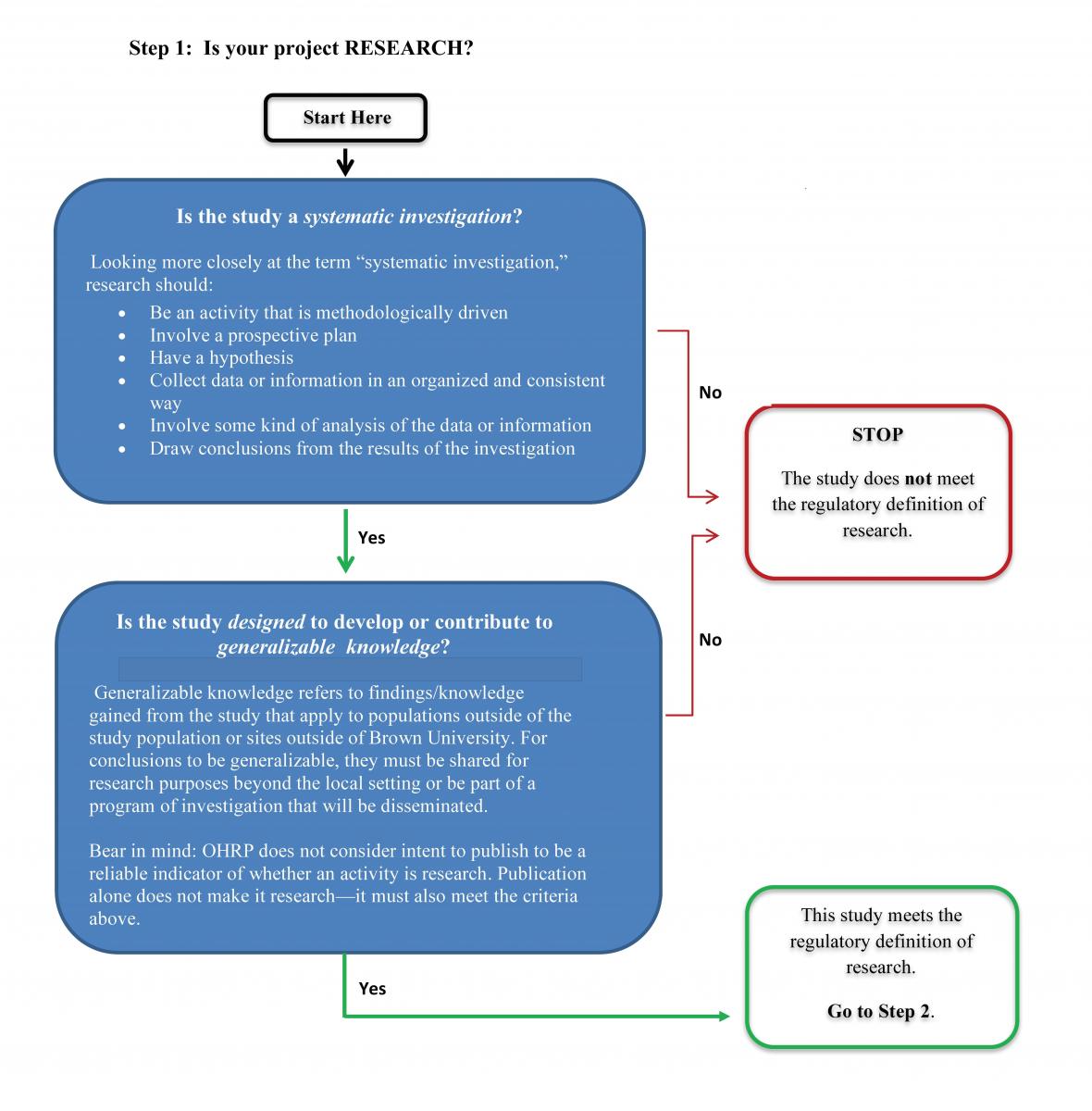
Does My Project Need IRB Review?, Research at Brown
Recomendado para você
-
IRBSL - ♥poussin♥☺Sidi Lakhdar21 março 2025
-
 صورة اخرى لنادي شبتين بعد حصوله على المركز الاول في دوري دير ابو مشعل 14\1\2011 - Shabtin - Ramallah - شبتين / شبطين (שיבתין) - Palestine Remembered21 março 2025
صورة اخرى لنادي شبتين بعد حصوله على المركز الاول في دوري دير ابو مشعل 14\1\2011 - Shabtin - Ramallah - شبتين / شبطين (שיבתין) - Palestine Remembered21 março 2025 -
 Casa com 4 dormitórios à venda, 141 m² por R$ 210.000,00 - S21 março 2025
Casa com 4 dormitórios à venda, 141 m² por R$ 210.000,00 - S21 março 2025 -
 About us - IRB21 março 2025
About us - IRB21 março 2025 -
 DJ TIGER Bodeli Chota Udepur Gujarat Demo Check Roadshow Sound System Setup Sharpy Lasers Truss21 março 2025
DJ TIGER Bodeli Chota Udepur Gujarat Demo Check Roadshow Sound System Setup Sharpy Lasers Truss21 março 2025 -
 Institute for Local Self-Reliance – Building Community, Strengthening Economies21 março 2025
Institute for Local Self-Reliance – Building Community, Strengthening Economies21 março 2025 -
 HISTÓRIA – IRBSL21 março 2025
HISTÓRIA – IRBSL21 março 2025 -
 Pew report: PA less restrictive on religion than Israel; Iran slightly worse21 março 2025
Pew report: PA less restrictive on religion than Israel; Iran slightly worse21 março 2025 -
 Global recognition – RSB21 março 2025
Global recognition – RSB21 março 2025 -
 Hydraulic Grease Fitting Kit, 32Pcs M6/M8/M10 Durable Brass Zerk Grease Nipple Fitting Assortment Kits with 2Pcs Grease Gun Pointy Tips21 março 2025
Hydraulic Grease Fitting Kit, 32Pcs M6/M8/M10 Durable Brass Zerk Grease Nipple Fitting Assortment Kits with 2Pcs Grease Gun Pointy Tips21 março 2025
você pode gostar
-
 Characters appearing in The Legendary Hero Is Dead! Anime21 março 2025
Characters appearing in The Legendary Hero Is Dead! Anime21 março 2025 -
 D. Gray-Man: Season One, Part One Blu-ray21 março 2025
D. Gray-Man: Season One, Part One Blu-ray21 março 2025 -
 Tokyo Ghoul - Ken Kaneki Wallpaper Download21 março 2025
Tokyo Ghoul - Ken Kaneki Wallpaper Download21 março 2025 -
 22 peças decorações de bolo de borboleta com toppers de bolo Acrílic feliz aniversário Para chuveiro do Bebê decoração da festa do aniversário do casamento (rosa roxo) - China Aniversário Party Decoration21 março 2025
22 peças decorações de bolo de borboleta com toppers de bolo Acrílic feliz aniversário Para chuveiro do Bebê decoração da festa do aniversário do casamento (rosa roxo) - China Aniversário Party Decoration21 março 2025 -
 Everyone Forgot About Roblox Trading Cards!?21 março 2025
Everyone Forgot About Roblox Trading Cards!?21 março 2025 -
 Forza Motorsport: Release date, platforms, gameplay & what we know so far21 março 2025
Forza Motorsport: Release date, platforms, gameplay & what we know so far21 março 2025 -
 Pin on Pins creados por ti21 março 2025
Pin on Pins creados por ti21 março 2025 -
 Is laser gun rare in MM2?21 março 2025
Is laser gun rare in MM2?21 março 2025 -
 ArtStation - Boruto - Chapter 64 (Rereading).21 março 2025
ArtStation - Boruto - Chapter 64 (Rereading).21 março 2025 -
Cleveland browns wallpaper by TheNatural22x - Download on ZEDGE™21 março 2025
