Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV
Por um escritor misterioso
Last updated 04 janeiro 2025


Inovio Pharmaceuticals, Inc. - INOVIO Announces Publication of Phase 1 Data from its COVID-19 DNA Vaccine Candidate, INO-4800 in The Lancet's EClinicalMedicine
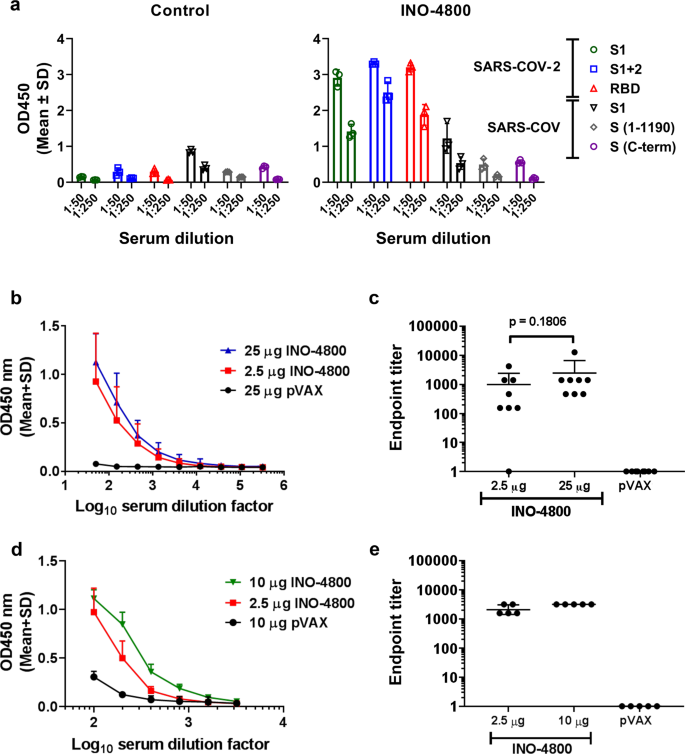
Immunogenicity of a DNA vaccine candidate for COVID-19

Positive Results from Preclinical Testing Support Clinical Development of COVID-19 DNA Vaccine
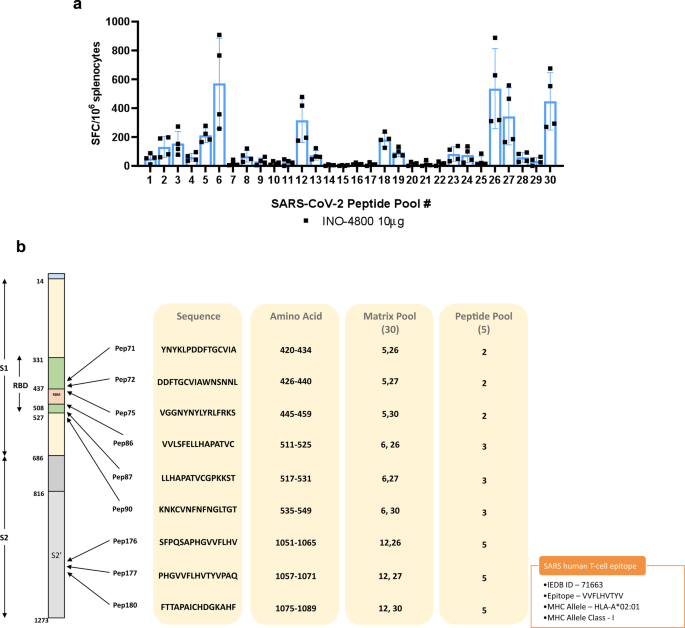
Immunogenicity of a DNA vaccine candidate for COVID-19
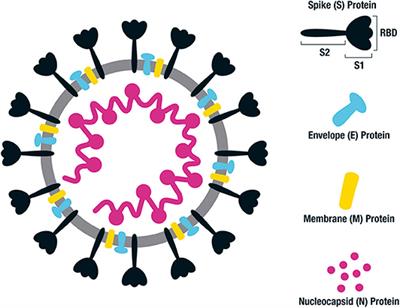
Immunogenicity of a DNA vaccine candidate for COVID-19
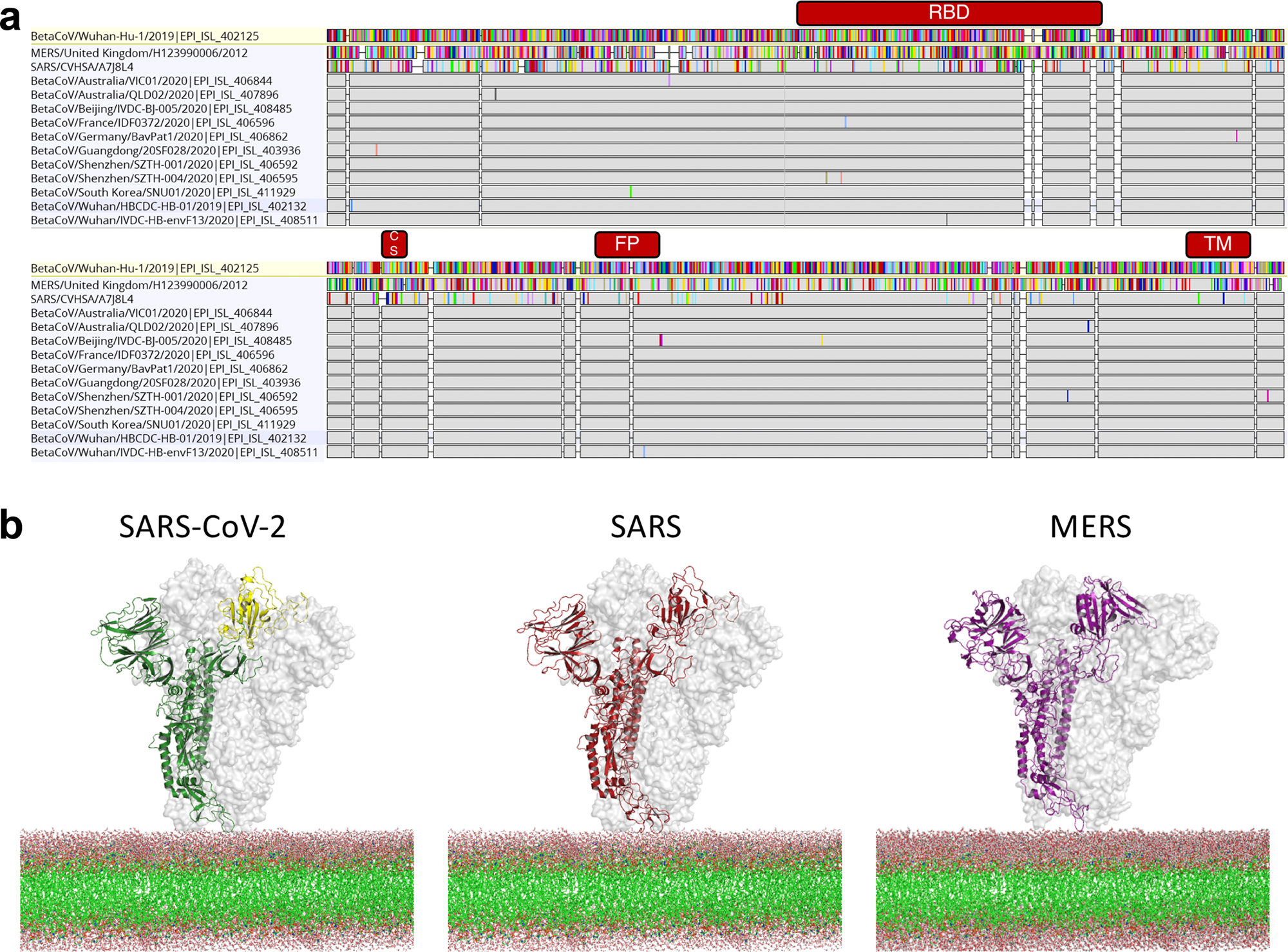
Frontiers Progress and Pitfalls in the Quest for Effective SARS-CoV-2 (COVID-19) Vaccines
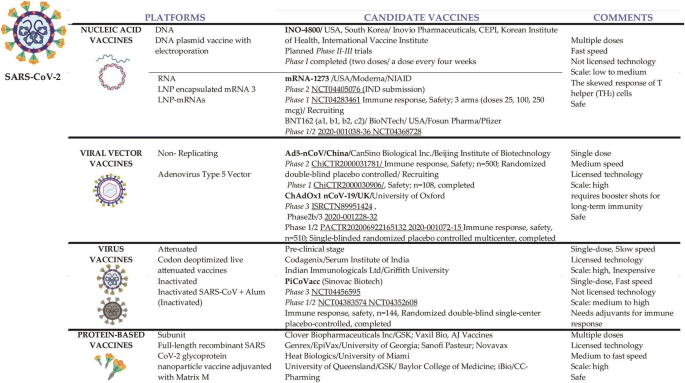
Recent advances, approaches and challenges in targeting pathways for potential COVID-19 vaccines development

A brief review on DNA vaccines in the era of COVID-19
Evaluation of a synthetic DNA SARS-CoV-2 vaccine INO-4800 using a nonhuman primate model

COVID-eVax, an electroporated DNA vaccine candidate encoding the SARS-CoV-2 RBD, elicits protective responses in animal models - ScienceDirect
Recomendado para você
-
 Learn About Dual Investment & Complete a Quiz to Receive a Dual04 janeiro 2025
Learn About Dual Investment & Complete a Quiz to Receive a Dual04 janeiro 2025 -
 Learning From COVID: Disruptions Shape Employer Expectations and04 janeiro 2025
Learning From COVID: Disruptions Shape Employer Expectations and04 janeiro 2025 -
 Binance Africa (@BinanceAfrica) / X04 janeiro 2025
Binance Africa (@BinanceAfrica) / X04 janeiro 2025 -
 Blueprint - NCEE04 janeiro 2025
Blueprint - NCEE04 janeiro 2025 -
:max_bytes(150000):strip_icc()/banking.asp-Final-e3a67ff9762b40aeac56983c22695032.jpg) The Evolution of Banking Over Time04 janeiro 2025
The Evolution of Banking Over Time04 janeiro 2025 -
 Self-Directed Investing: Online Stock Trading On Your Terms04 janeiro 2025
Self-Directed Investing: Online Stock Trading On Your Terms04 janeiro 2025 -
 Comparing characteristics and selected expenditures of dual- and04 janeiro 2025
Comparing characteristics and selected expenditures of dual- and04 janeiro 2025 -
 Lanier Technical College04 janeiro 2025
Lanier Technical College04 janeiro 2025 -
 Learn About Dual Investment & Complete a Quiz to Receive a Dual04 janeiro 2025
Learn About Dual Investment & Complete a Quiz to Receive a Dual04 janeiro 2025 -
 The Ultimate Guide to Customer Education in SaaS: Best Practices04 janeiro 2025
The Ultimate Guide to Customer Education in SaaS: Best Practices04 janeiro 2025
você pode gostar
-
 The Entity (film version), Elm Street Wiki04 janeiro 2025
The Entity (film version), Elm Street Wiki04 janeiro 2025 -
 Cavaleiros do Zodíaco ganha novas vozes após crise na dublagem, entenda a treta04 janeiro 2025
Cavaleiros do Zodíaco ganha novas vozes após crise na dublagem, entenda a treta04 janeiro 2025 -
 Persian Cat For Sale : Buy Persian Kittens Online At Best Price04 janeiro 2025
Persian Cat For Sale : Buy Persian Kittens Online At Best Price04 janeiro 2025 -
 Top Gun Maverick Soundtrack CD04 janeiro 2025
Top Gun Maverick Soundtrack CD04 janeiro 2025 -
 Classroom of The Elite Season 3 Release Date, Trailer, Plot, and04 janeiro 2025
Classroom of The Elite Season 3 Release Date, Trailer, Plot, and04 janeiro 2025 -
 Esports Awards 2023: Schedule, Awards, Nominees04 janeiro 2025
Esports Awards 2023: Schedule, Awards, Nominees04 janeiro 2025 -
Torin 2 Ton Capacity 89 in. Lift Big Red Engine Hoist at Tractor04 janeiro 2025
-
Dark ZerO Gaming - Artist: Zumi Character: Cammy (Street Fighter 6) #Cammy # SF6 #StreetFighter6 #Capcom #Art #Zumi04 janeiro 2025
-
 Paciente chega de madrugada para pegar ficha em UBS e mesmo assim não consegue04 janeiro 2025
Paciente chega de madrugada para pegar ficha em UBS e mesmo assim não consegue04 janeiro 2025 -
 How to Watch ONE PIECE Faster Without Filler Episodes - Nerdist04 janeiro 2025
How to Watch ONE PIECE Faster Without Filler Episodes - Nerdist04 janeiro 2025

