FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 07 julho 2024

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services
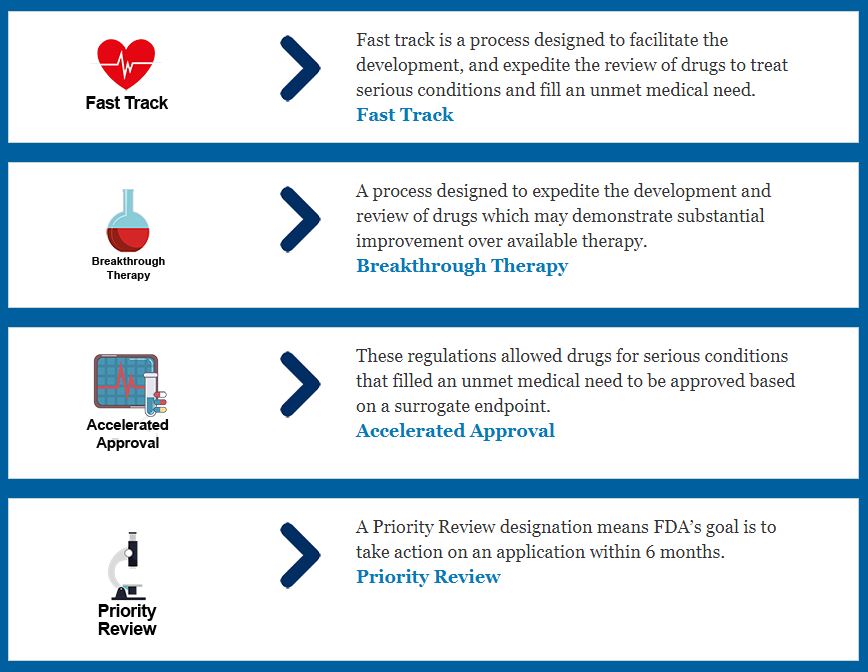
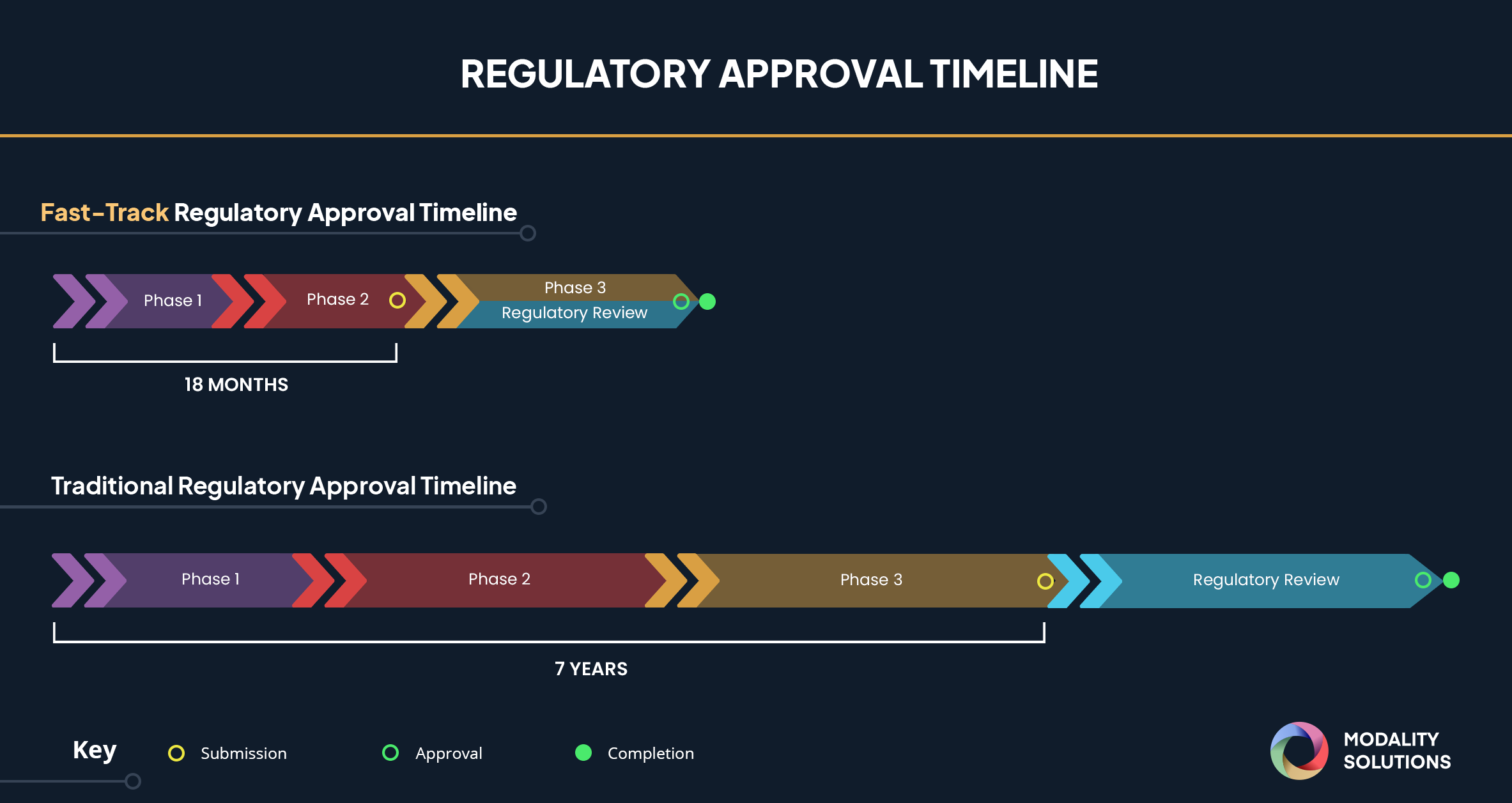
Drug Development Process

FDA's Fast-Track for Rexulti Raises Concerns
Rexulti: Uses, Side Effects & Warnings
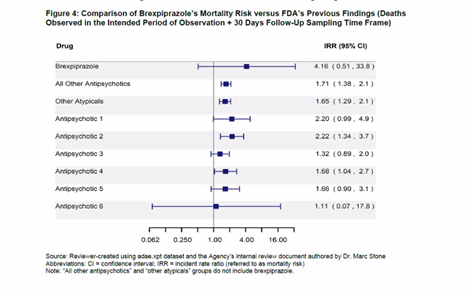
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

FDA rushes approval of dementia drug that quadruples risk of death

FDA fast-tracked dementia drug with known harms, reporter says - Clinical Daily News - McKnight's Long-Term Care News

FDA's Fast-Track for Rexulti Raises Concerns
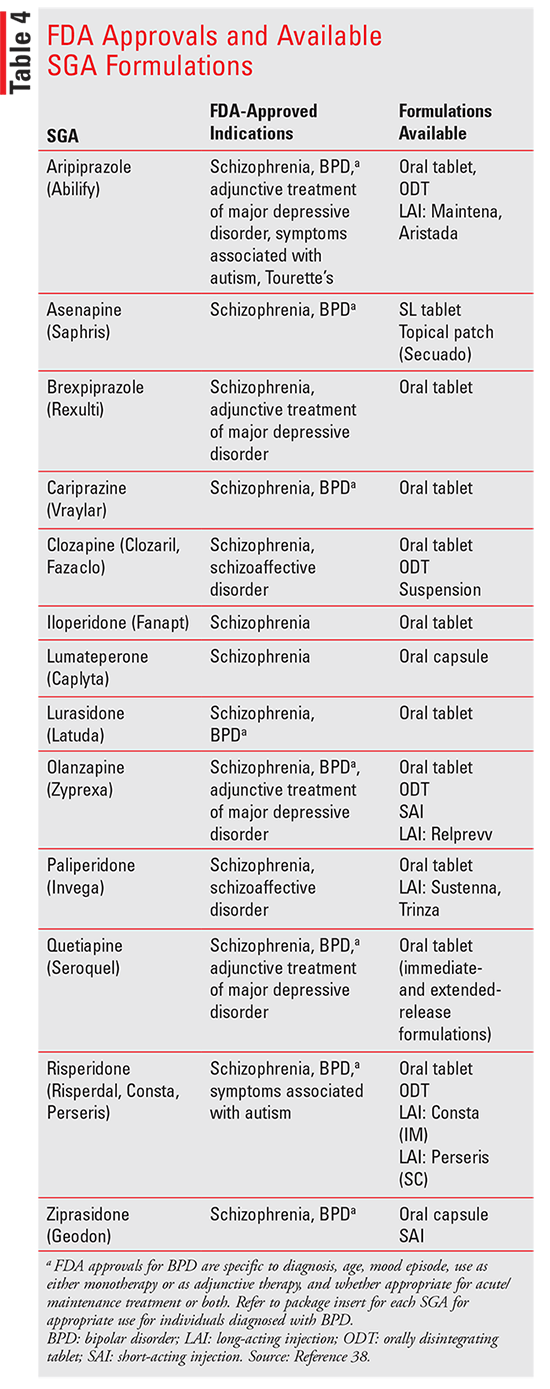
Lesson: Assessing the Current Antipsychotics Landscape

Video: Depression is a Journey

FDA rushes approval of dementia drug that quadruples risk of death

FDA fast-tracks drug for dementia agitation that increases risk of death 4 times - Study Finds
FDA's Fast Track Approval Coronavirus Treatment Acceleration Program

FDA's Fast Track Approval Coronavirus Treatment Acceleration Program
Recomendado para você
-
 Rexulti 1 mg 28 tablets07 julho 2024
Rexulti 1 mg 28 tablets07 julho 2024 -
 Buy Rexulti (Brexpiprazole) from Our Certified Canadian Pharmacy07 julho 2024
Buy Rexulti (Brexpiprazole) from Our Certified Canadian Pharmacy07 julho 2024 -
 Been in a depressive episode for too long doctor added rexulti to the mix did any of you have any luck with it ? : r/bipolar207 julho 2024
Been in a depressive episode for too long doctor added rexulti to the mix did any of you have any luck with it ? : r/bipolar207 julho 2024 -
 How Marketers Accentuate Clinical Messaging07 julho 2024
How Marketers Accentuate Clinical Messaging07 julho 2024 -
 New Drug Product: REXULTI - MPR07 julho 2024
New Drug Product: REXULTI - MPR07 julho 2024 -
 Compra Rexulti brexpiprazol 2 mg con 14 tabletas en Prixz07 julho 2024
Compra Rexulti brexpiprazol 2 mg con 14 tabletas en Prixz07 julho 2024 -
 Rexulti Patient Site - Once Daily Pharma07 julho 2024
Rexulti Patient Site - Once Daily Pharma07 julho 2024 -
 Rexulti - 2 mg - 28 tablets07 julho 2024
Rexulti - 2 mg - 28 tablets07 julho 2024 -
 REXULTI 2 MG Oral Tablet07 julho 2024
REXULTI 2 MG Oral Tablet07 julho 2024 -
 Pharmacy: Rexulti (Brand for Brexpiprazole, Oral Tablet)07 julho 2024
Pharmacy: Rexulti (Brand for Brexpiprazole, Oral Tablet)07 julho 2024
você pode gostar
-
 Pixilart - Cursed Piggy Ship by CloudMoostache07 julho 2024
Pixilart - Cursed Piggy Ship by CloudMoostache07 julho 2024 -
 Assistir Yu-Gi-Oh! Dublado Episodio 129 Online07 julho 2024
Assistir Yu-Gi-Oh! Dublado Episodio 129 Online07 julho 2024 -
 Gotta die a lot: how to artificially raise the difficulty in07 julho 2024
Gotta die a lot: how to artificially raise the difficulty in07 julho 2024 -
 Comprar Stray PS4 - Isagui Games 12 Anos a Melhor Loja de Jogos07 julho 2024
Comprar Stray PS4 - Isagui Games 12 Anos a Melhor Loja de Jogos07 julho 2024 -
2K anuncia que vai lançar novo jogo da NHL em breve07 julho 2024
-
 Show by Rock!!/#1908742 Anime, Rock, Anime shows07 julho 2024
Show by Rock!!/#1908742 Anime, Rock, Anime shows07 julho 2024 -
 The Lords of the Fallen Announcement Trailer07 julho 2024
The Lords of the Fallen Announcement Trailer07 julho 2024 -
Tenjou Tenge s2 Episode 1, Tenjou Tenge s2, By Anime.com07 julho 2024
-
 Jogo de Tabuleiro Pac-Man The Board Game « Blog de Brinquedo07 julho 2024
Jogo de Tabuleiro Pac-Man The Board Game « Blog de Brinquedo07 julho 2024 -
 Lolbit FNAF Funko Figure07 julho 2024
Lolbit FNAF Funko Figure07 julho 2024
