Early Safety Assessment - Drug Discovery and Development Based on
Por um escritor misterioso
Last updated 04 outubro 2024

The drug candidate faces numerous efficacy and safety hurdles before moving forward to clinical testing. Here at the UPDDI we recognize the need for early identification of potential human toxicity and pharmacokinetic issues by creating a unique human liver microphysiological systems platform for drug testing before implementing preclinical animal testing.
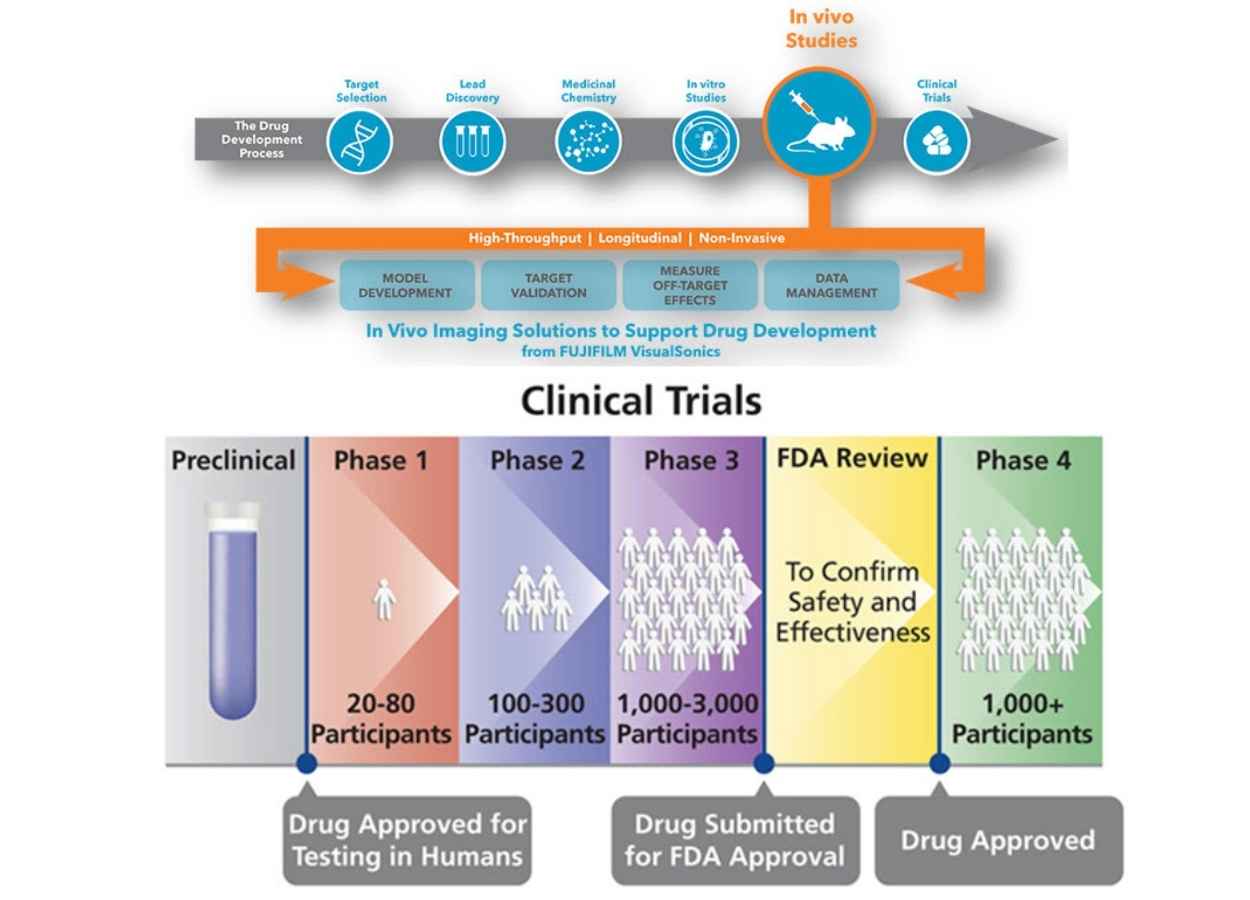
AN OVERVIEW OF NEW DRUG DISCOVERY AND DEVELOPMENT
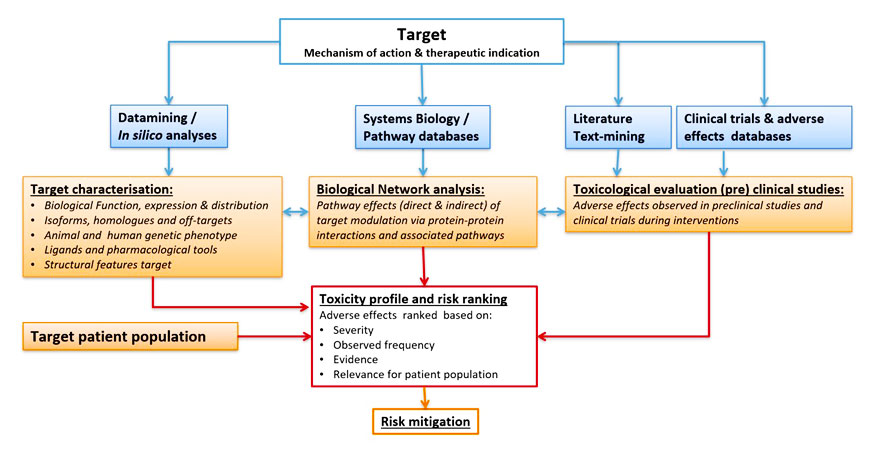
Target Profiling

Target Safety Assessments And Their Role In De-risking Drug Discovery
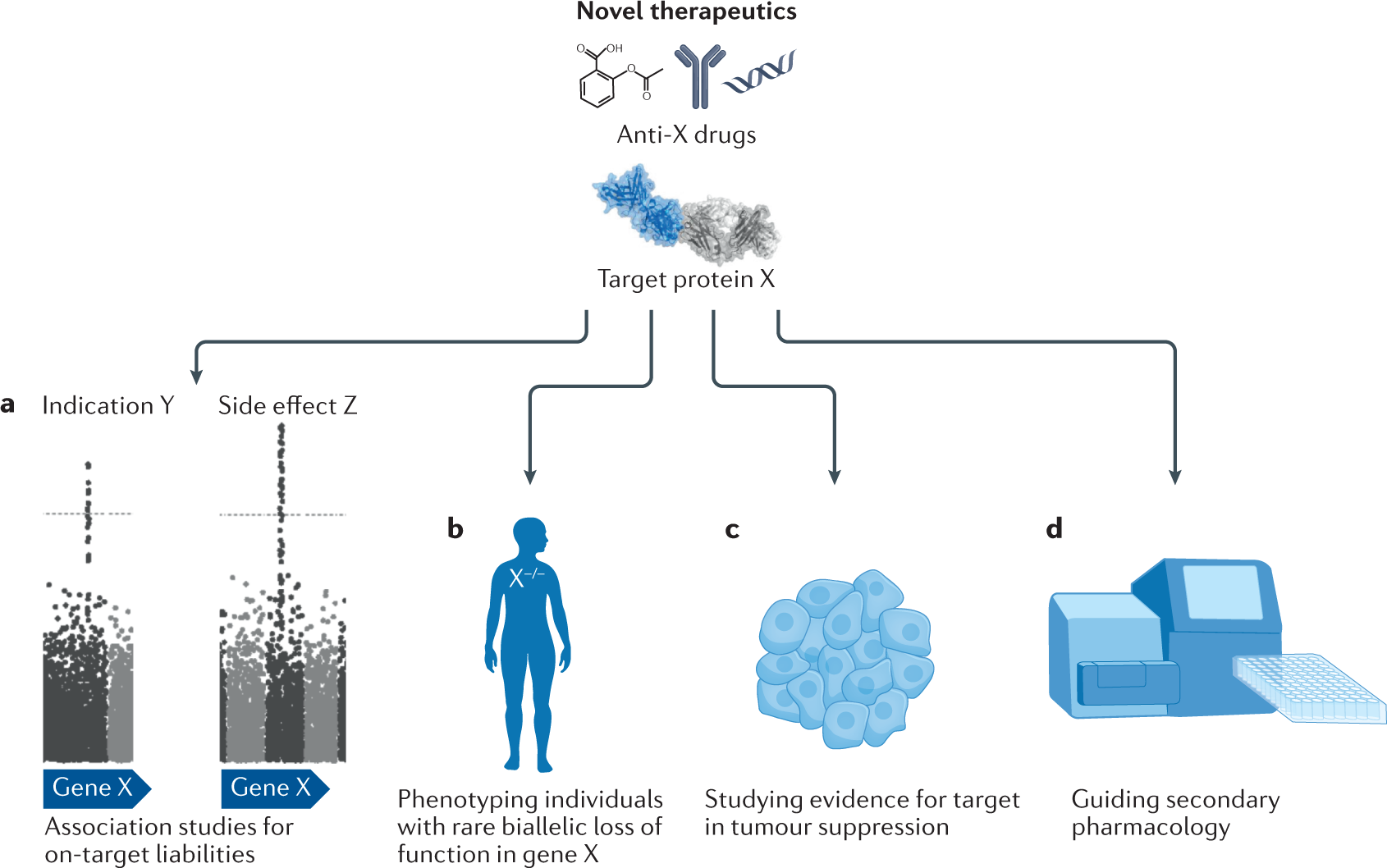
Using human genetics to improve safety assessment of therapeutics

Drug discovery - Wikipedia

Drug development - Wikipedia

Eurofins Advinus BioPharma Services - Eurofins Scientific

Drug Discovery and Development: An Overview - ScienceDirect

Rational steps for drug discovery and development.

Preclinical safety evaluation of biotechnology-derived
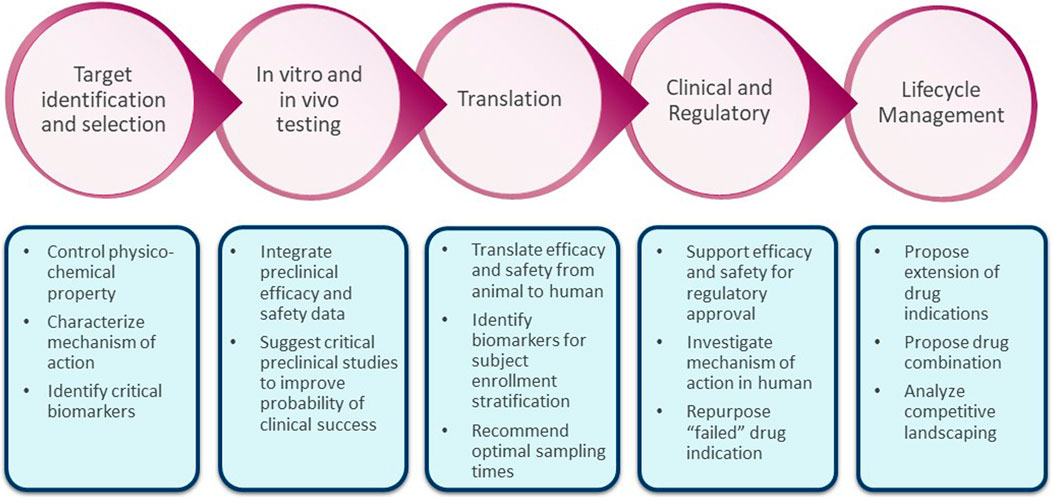
Frontiers Quantitative systems modeling approaches towards model

PDF) Investigative safety strategies to improve success in drug
Recomendado para você
-
 BRAIN TEST LEVEL 411 WALK THROUGH WITH COMMENTARY04 outubro 2024
BRAIN TEST LEVEL 411 WALK THROUGH WITH COMMENTARY04 outubro 2024 -
The University of Alabama's Brain-Drone Race Flies Us to a Mind04 outubro 2024
-
 EarthBound / Mother 3 Goodness.04 outubro 2024
EarthBound / Mother 3 Goodness.04 outubro 2024 -
 Number Cross: 200 Number Cross Puzzles Designed to keep your brain04 outubro 2024
Number Cross: 200 Number Cross Puzzles Designed to keep your brain04 outubro 2024 -
CONSORT diagram. CCT conventional coagulation test, VHA04 outubro 2024
-
 Classification of autism spectrum disorder based on sample entropy04 outubro 2024
Classification of autism spectrum disorder based on sample entropy04 outubro 2024 -
 Got the pink shorthair! : r/CatsAndSoup04 outubro 2024
Got the pink shorthair! : r/CatsAndSoup04 outubro 2024 -
 Brain Training – Ginger Fox04 outubro 2024
Brain Training – Ginger Fox04 outubro 2024 -
 Studies add details about the brain, clues for future treatments04 outubro 2024
Studies add details about the brain, clues for future treatments04 outubro 2024 -
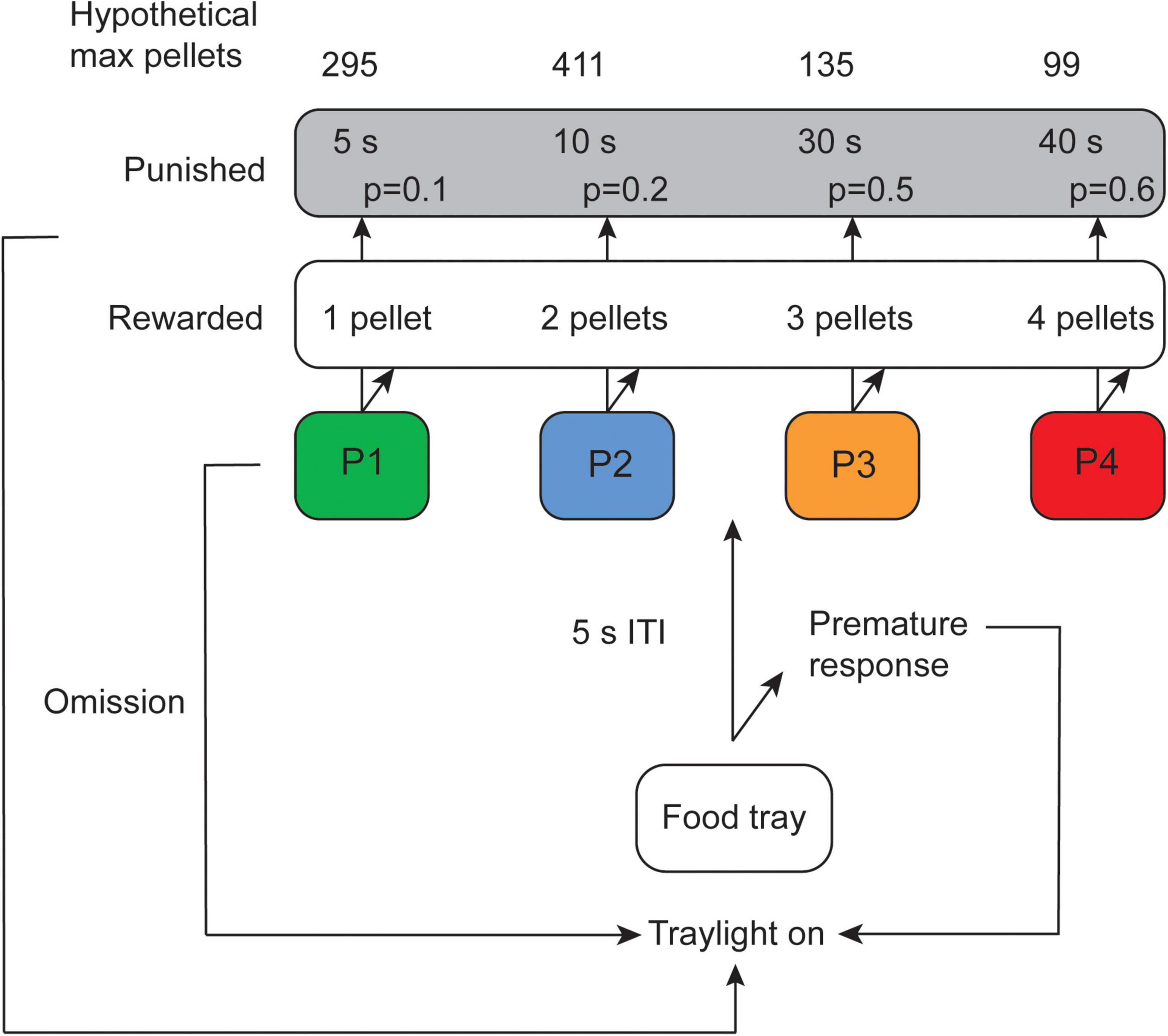 Frontiers Exploring decision-making strategies in the Iowa04 outubro 2024
Frontiers Exploring decision-making strategies in the Iowa04 outubro 2024
você pode gostar
-
 Game Bắn súng Online 2 - Aim Clash 2 - Game Vui04 outubro 2024
Game Bắn súng Online 2 - Aim Clash 2 - Game Vui04 outubro 2024 -
 Blocos de Montar - Cidade - Ambulância - Carro de Fricção - 5404 outubro 2024
Blocos de Montar - Cidade - Ambulância - Carro de Fricção - 5404 outubro 2024 -
 Add pirated DLC's to your legal The Sims 4 Game for free (Windows only) < The Sims free downloads for windows04 outubro 2024
Add pirated DLC's to your legal The Sims 4 Game for free (Windows only) < The Sims free downloads for windows04 outubro 2024 -
 clash royale laugh by RodolfoLSF Sound Effect - Meme Button - Tuna04 outubro 2024
clash royale laugh by RodolfoLSF Sound Effect - Meme Button - Tuna04 outubro 2024 -
 Com a benção do Papai Noel – Décimo Andar04 outubro 2024
Com a benção do Papai Noel – Décimo Andar04 outubro 2024 -
O começo da Guerra Naruto Shippuden Episódio 262 parte 03 . . #Cap04 outubro 2024
-
 Dungeon ni Deai: Arrow of the Orion – Um ótimo filme para os fãs04 outubro 2024
Dungeon ni Deai: Arrow of the Orion – Um ótimo filme para os fãs04 outubro 2024 -
 Hand-forged Kratos Axe Fully Functional Leviathan Axe04 outubro 2024
Hand-forged Kratos Axe Fully Functional Leviathan Axe04 outubro 2024 -
 Plants Vs. Zombies Limited Sunflower Edition Sealed RARE04 outubro 2024
Plants Vs. Zombies Limited Sunflower Edition Sealed RARE04 outubro 2024 -
 The last of us (tv series) Wallpaper04 outubro 2024
The last of us (tv series) Wallpaper04 outubro 2024