Duragesic: Package Insert
Por um escritor misterioso
Last updated 21 março 2025

Duragesic package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology.

Transdermal Patches: How to Apply Them

WO2002074286A1 - Transdermal patch for administering fentanyl - Google Patents
Duragesic Medicare Coverage and Co-Pay Details - GoodRx
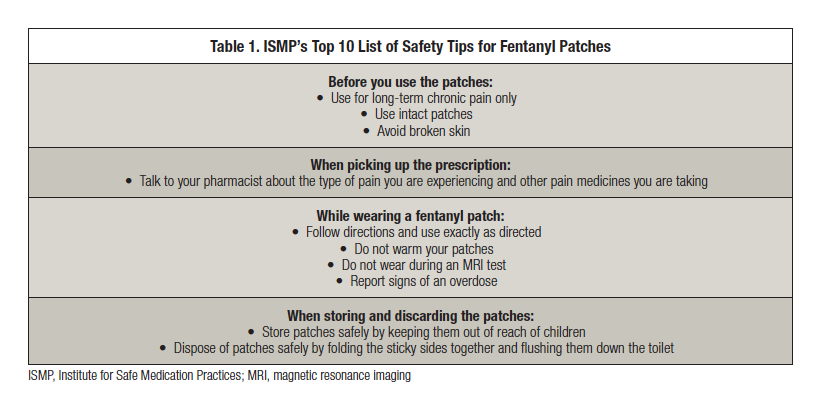
Proper Disposal of Fentanyl Patches: What Patients Need to Know

Opioid Analgesics for Persistent Pain in the Older Patient: Part II

FentaNYL Patch Not Adhering Properly During Use, Article
FENTANYL TRANSDERMAL SYSTEM 25 mcg/hr, 50 mcg/hr, 75 mcg/hr, 100 mcg/hr CII Full Prescribing Information Rx only FOR USE IN OPIOID-TOLERANT PATIENTS ONLY

Duragesic Snapper - Information Packaging

Inpatient Prescribing and Monitoring of Fentanyl Transdermal Systems: Adherence to Safety Regulations - Theresa McEvoy, Joann Moore, Joyce Generali, 2014
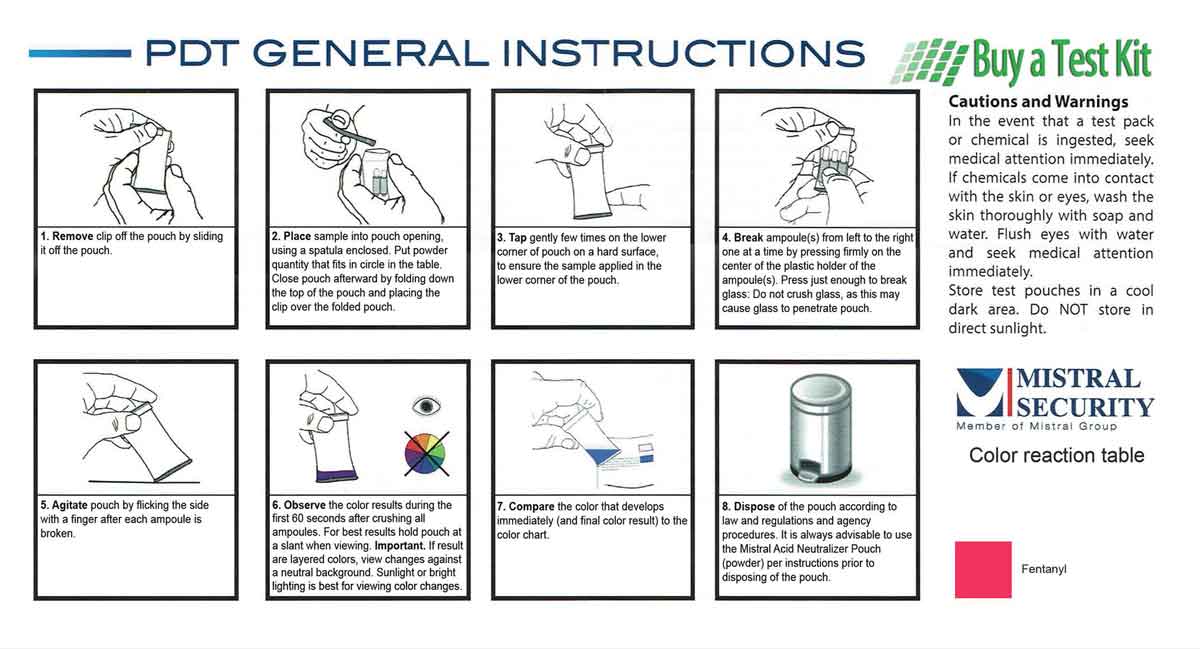
A Fentanyl Test Kit to Detect and Identify Fentanyl (2 pack)

Can You Cut Transdermal Patches in Half? - GoodRx
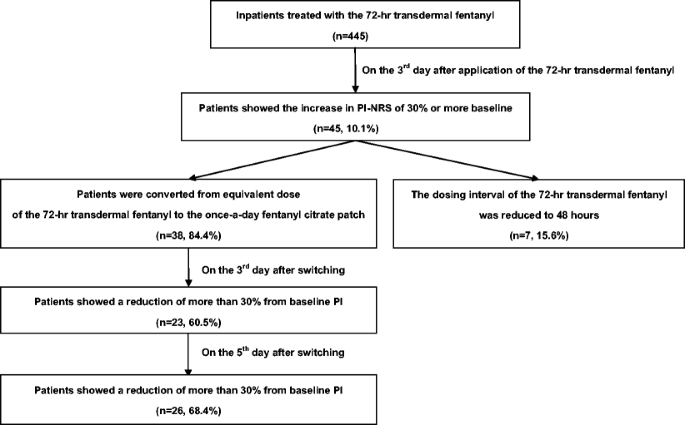
A new once-a-day fentanyl citrate patch (Fentos® Tape) could be a new treatment option in patients with end-of-dose failure using a 72-h transdermal fentanyl matrix patch
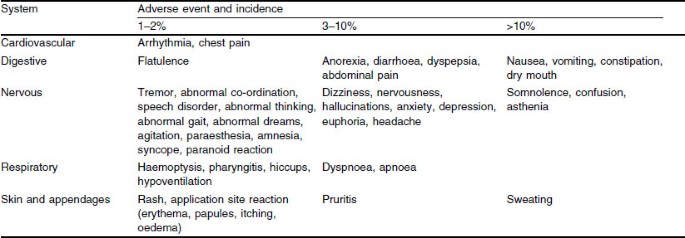
Benefit-Risk Assessment of Transdermal Fentanyl for the Treatment of Chronic Pain
Recomendado para você
-
 Durogesic 50mcg - Hillside Veterinary Surgery21 março 2025
Durogesic 50mcg - Hillside Veterinary Surgery21 março 2025 -
 Durogesic 50mcg Patch, Treatment: Pain Relief, Janssen21 março 2025
Durogesic 50mcg Patch, Treatment: Pain Relief, Janssen21 março 2025 -
 File:Durogesic 50ug plasters.jpg - Wikimedia Commons21 março 2025
File:Durogesic 50ug plasters.jpg - Wikimedia Commons21 março 2025 -
 Durogesic Matrixpfl 25 mcg/h 5 Stk mit Rezept online bestellen21 março 2025
Durogesic Matrixpfl 25 mcg/h 5 Stk mit Rezept online bestellen21 março 2025 -
 Durogesic hi-res stock photography and images - Alamy21 março 2025
Durogesic hi-res stock photography and images - Alamy21 março 2025 -
RACGP - Organised crime targeting GPs for potent fentanyl patches21 março 2025
-
 transdermal patch Products - transdermal patch Manufacturers, Exporters, Suppliers on EC21 Mobile21 março 2025
transdermal patch Products - transdermal patch Manufacturers, Exporters, Suppliers on EC21 Mobile21 março 2025 -
 Durogesic Pl Emp 10x 12mcg/heure/uur21 março 2025
Durogesic Pl Emp 10x 12mcg/heure/uur21 março 2025 -
 Durogesic (50 mg.) – Redconac21 março 2025
Durogesic (50 mg.) – Redconac21 março 2025 -
 Durogesic D-Trans 50mcg/h 5 Adesivos Transdérmicos - Durogesic D-Trans 50mcg/h 5 Adesivos Transdérmicos - JANSSEN-CILAG21 março 2025
Durogesic D-Trans 50mcg/h 5 Adesivos Transdérmicos - Durogesic D-Trans 50mcg/h 5 Adesivos Transdérmicos - JANSSEN-CILAG21 março 2025
você pode gostar
-
 Faça já seu plano TIM controle em Carpina e aproveite as super vantagens21 março 2025
Faça já seu plano TIM controle em Carpina e aproveite as super vantagens21 março 2025 -
 NEW* UPDATE! CODES* 🏆 Race Clicker ROBLOX21 março 2025
NEW* UPDATE! CODES* 🏆 Race Clicker ROBLOX21 março 2025 -
Mahoutsukai no Yome Season 2 Episode 121 março 2025
-
 Standoff 2 Private Server 6.5 🔥 (Anton Snak) Download Via21 março 2025
Standoff 2 Private Server 6.5 🔥 (Anton Snak) Download Via21 março 2025 -
 Sayonara Wild Hearts - Wikipedia21 março 2025
Sayonara Wild Hearts - Wikipedia21 março 2025 -
 Explore the Crown Tundra with the #PokemonSwordShield Expansion21 março 2025
Explore the Crown Tundra with the #PokemonSwordShield Expansion21 março 2025 -
:quality(80)/lojaiiie/catalog/mochilas/mochilas-personagens/248.png) Mochila Infantil Grande Sonic Feito Para Correr - Preto21 março 2025
Mochila Infantil Grande Sonic Feito Para Correr - Preto21 março 2025 -
 reactions on X: smug stick figure holding up nordic chad meme nice try defending your point too bad I've made this meme where you're the ugly face and I'm the handsome one21 março 2025
reactions on X: smug stick figure holding up nordic chad meme nice try defending your point too bad I've made this meme where you're the ugly face and I'm the handsome one21 março 2025 -
 CapCut_invencível 2 temporada episódio 221 março 2025
CapCut_invencível 2 temporada episódio 221 março 2025 -
 Gun Box 3, Murder Mystery 2 Wiki21 março 2025
Gun Box 3, Murder Mystery 2 Wiki21 março 2025