4C2U: Crystal Structure Of Deinococcus Radiodurans Uvrd In Complex
Por um escritor misterioso
Last updated 09 março 2025
DNA HELICASE IIFOR25REV25Glycerin; Propane-1,2,3-triolGlycerolMagnesium IonNitrate IonPhosphoaminophosphonic Acid-Adenylate EsterSodium Ion

The structured organization of Deinococcus radiodurans' cell envelope
Crucial Roles of Carotenoids as Bacterial Endogenous Defense System for Bacterial Radioresistance of Deinococcus radiodurans
RCSB PDB - 7QVB: Crystal structure of the DNA-binding protein DdrC from Deinococcus radiodurans
RCSB PDB - 4Q0J: Deinococcus radiodurans BphP photosensory module
RCSB PDB - 7QVB: Crystal structure of the DNA-binding protein DdrC from Deinococcus radiodurans

Surface (S)-layer proteins of Deinococcus radiodurans and their utility as vehicles for surface localization of functional proteins - ScienceDirect

4C2U: Crystal Structure Of Deinococcus Radiodurans Uvrd In Complex With Dna, Form 1
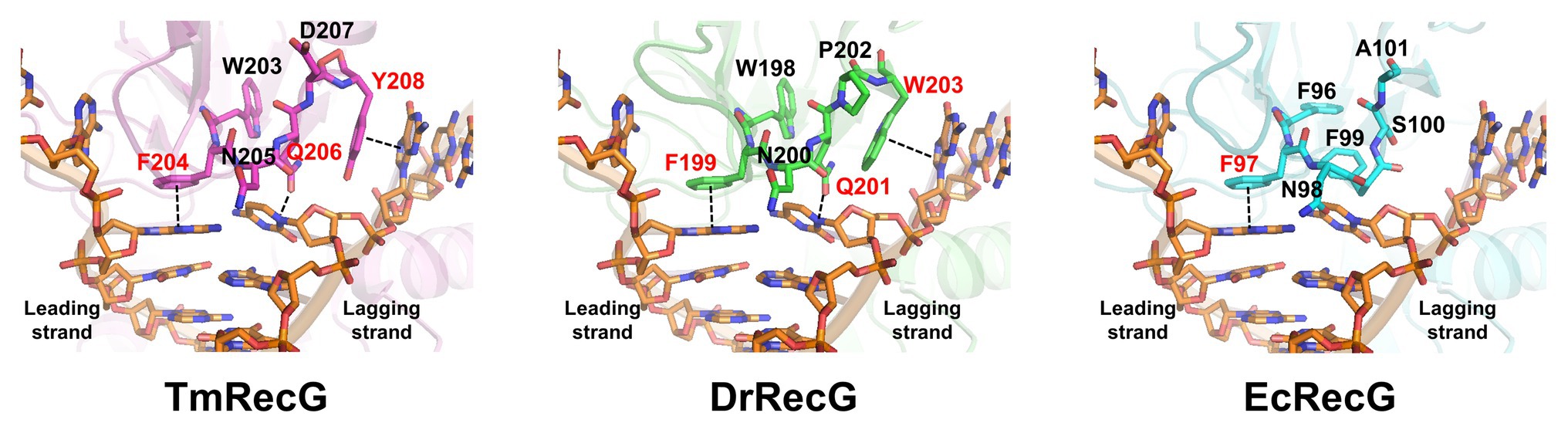
Frontiers Effects of Conserved Wedge Domain Residues on DNA Binding Activity of Deinococcus radiodurans RecG Helicase

PDF) Structural features and functional implications of proteins enabling the robustness of Deinococcus radiodurans
4C2U: Crystal Structure Of Deinococcus Radiodurans Uvrd In Complex With Dna, Form 1
Recomendado para você
-
 Deinococcus Radiodurans: The World's Toughest Bacterium. A Review09 março 2025
Deinococcus Radiodurans: The World's Toughest Bacterium. A Review09 março 2025 -
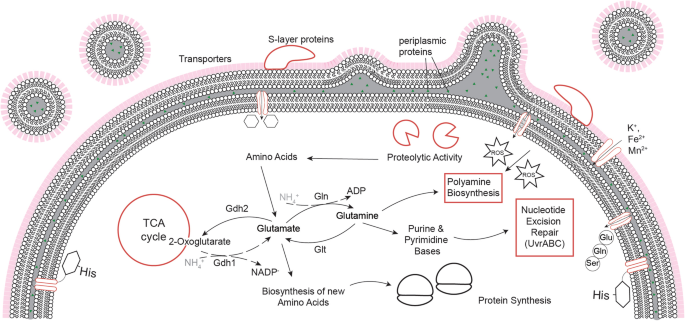 Molecular repertoire of Deinococcus radiodurans after 1 year of09 março 2025
Molecular repertoire of Deinococcus radiodurans after 1 year of09 março 2025 -
 Deinococcus radiodurans09 março 2025
Deinococcus radiodurans09 março 2025 -
 Colonized extremophile Deinococcus radiodurans alleviates toxicity09 março 2025
Colonized extremophile Deinococcus radiodurans alleviates toxicity09 março 2025 -
 Genome Sequence of the Radioresistant Bacterium Deinococcus09 março 2025
Genome Sequence of the Radioresistant Bacterium Deinococcus09 março 2025 -
 The RecA proteins of Deinococcus radiodurans and Escherichia coli09 março 2025
The RecA proteins of Deinococcus radiodurans and Escherichia coli09 março 2025 -
 A tentative model of the S-layer of Deinococcus radiodurans R 109 março 2025
A tentative model of the S-layer of Deinococcus radiodurans R 109 março 2025 -
 Accumulation of Mn(II) in Deinococcus radiodurans Facilitates09 março 2025
Accumulation of Mn(II) in Deinococcus radiodurans Facilitates09 março 2025 -
 Deinococcus radiodurans09 março 2025
Deinococcus radiodurans09 março 2025 -
 Functionalized Nanomaterial Assembling and Biosynthesis Using the09 março 2025
Functionalized Nanomaterial Assembling and Biosynthesis Using the09 março 2025
você pode gostar
-
 Sony-PlayStation 5 Maneater PlayStation, PS 5 Game Disks, Ofertas para Plataforma - AliExpress09 março 2025
Sony-PlayStation 5 Maneater PlayStation, PS 5 Game Disks, Ofertas para Plataforma - AliExpress09 março 2025 -
 Believe in the Lie — Genshin Impact Tier List but it's based on the09 março 2025
Believe in the Lie — Genshin Impact Tier List but it's based on the09 março 2025 -
 Como ser um otaku : ANALISE : ROKUDENASHI MAJUTSU KOUSHI TO AKASHIC RECORDS09 março 2025
Como ser um otaku : ANALISE : ROKUDENASHI MAJUTSU KOUSHI TO AKASHIC RECORDS09 março 2025 -
Merely's Web Slinger Roblox Limited Item - Rolimon's09 março 2025
-
 Otonari no Tenshi-sama ni Itsunomanika Dame Ningen ni Sareteita Ken - Baka-Updates Manga09 março 2025
Otonari no Tenshi-sama ni Itsunomanika Dame Ningen ni Sareteita Ken - Baka-Updates Manga09 março 2025 -
 Hideo Kojima - Variety500 - Top 500 Entertainment Business Leaders09 março 2025
Hideo Kojima - Variety500 - Top 500 Entertainment Business Leaders09 março 2025 -
 otP Download Game Pokémon Online MMORPG09 março 2025
otP Download Game Pokémon Online MMORPG09 março 2025 -
 Athos (Minecraft)09 março 2025
Athos (Minecraft)09 março 2025 -
 Trio Peças De Xadrez Rei Torre Cavalo Cerâmica Clássica Branca09 março 2025
Trio Peças De Xadrez Rei Torre Cavalo Cerâmica Clássica Branca09 março 2025 -
 DVD ANIME BORUTO: NARUTO NEXT GENERATIONS Vol.280-293 REGION ALL ENGLISH SUBS09 março 2025
DVD ANIME BORUTO: NARUTO NEXT GENERATIONS Vol.280-293 REGION ALL ENGLISH SUBS09 março 2025