Life Sciences Commissioning, Qualification and Validation
Por um escritor misterioso
Last updated 09 janeiro 2025


A Basic Guide to IQ, OQ, PQ in FDA-Regulated Industries
Commissioning, Qualification and Validation (CQV) are requirements of modern facilities within the Life Science industry. Be it a Medical Device

Commissioning, Qualification and Validation: A GMP Approach
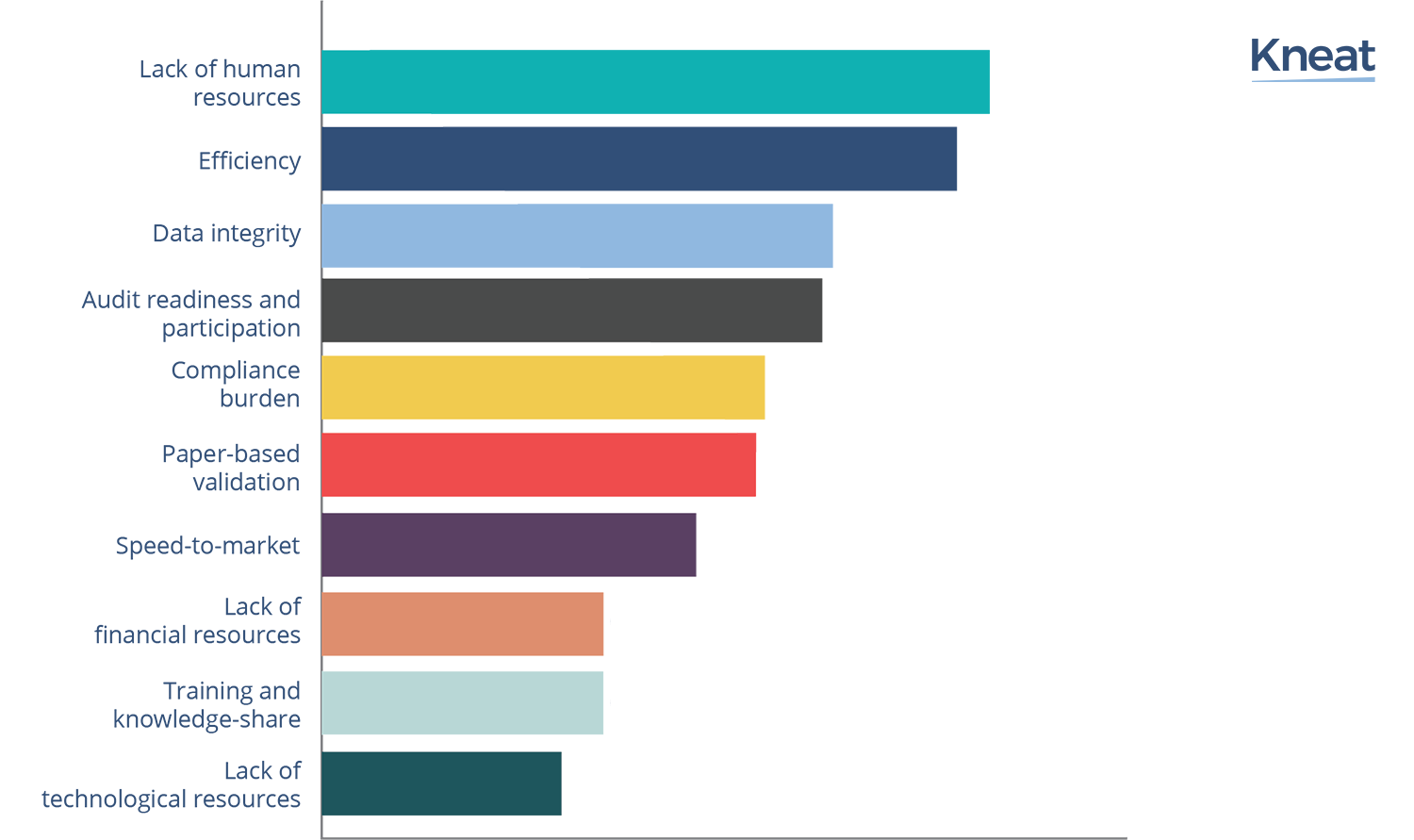
Top 10 Validation Challenges for Life Sciences SMBs in 2022 - Kneat

Commissioning, Qualification & Validation – Deci

Commissioning, Qualification and Validation (CQV) in Pharma

Commissioning Qualification and Validation the Concept of CQV – Part 2 - LearnGxP: Accredited Online Life Science Training Courses
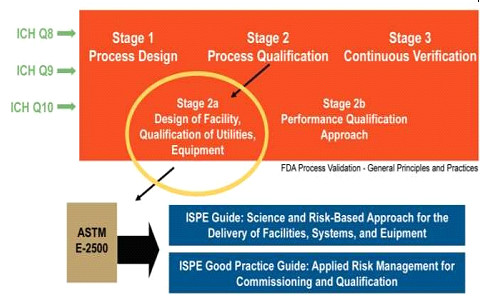
CQV

Commissioning and Qualification - VTI Life Sciences

Commissioning, Qualification and Validation: A GMP Approach , Browne, Priscilla

Commissioning & Qualification: Debunking the Myths – No deviation

Business Case: Commissioning, Qualification, and Validation (CQV) for Facility, Manufacturing, and Laboratory Equipment and Systems - Kvalito
Recomendado para você
-
 I just had to check ICQ project09 janeiro 2025
I just had to check ICQ project09 janeiro 2025 -
 Fall into Temptation: A Small Town Love Story (Blue09 janeiro 2025
Fall into Temptation: A Small Town Love Story (Blue09 janeiro 2025 -
![Oh Boy Green Baby Shower Invitation, Eucalyptus and Greenery Baby Shower Invite Template, Baby Shower Instant Download [id:4397601,4397781]](https://i.etsystatic.com/6760092/r/il/959756/2398614640/il_fullxfull.2398614640_4icq.jpg) Oh Boy Green Baby Shower Invitation, Eucalyptus and Greenery Baby Shower Invite Template, Baby Shower Instant Download [id:4397601,4397781]09 janeiro 2025
Oh Boy Green Baby Shower Invitation, Eucalyptus and Greenery Baby Shower Invite Template, Baby Shower Instant Download [id:4397601,4397781]09 janeiro 2025 -
 10 Websites and Services We Loved in the 90s09 janeiro 2025
10 Websites and Services We Loved in the 90s09 janeiro 2025 -
 F4.png09 janeiro 2025
F4.png09 janeiro 2025 -
 Classic ICQ : r/nostalgia09 janeiro 2025
Classic ICQ : r/nostalgia09 janeiro 2025 -
 Victor on X: Two screenshots. 🔸ICQ (~2000s) 🔸Telegram (~2020s) Did you notice how we went from having many windows to a single one? Settings, sending images/files, having many chats - everything in09 janeiro 2025
Victor on X: Two screenshots. 🔸ICQ (~2000s) 🔸Telegram (~2020s) Did you notice how we went from having many windows to a single one? Settings, sending images/files, having many chats - everything in09 janeiro 2025 -
![Cards Cloned/PAYPAL] WWW.Trusted-Best.bz Shop Dumps IST Pin/Card Clone SHOP, PRIVATE SNIFFER, BEST VALIDWe invite sellers,Welcome!, Emv Softwa](https://static.wixstatic.com/media/011f31_f0edb3ba705e4712b539b563e0e4f63e~mv2.png/v1/fill/w_520,h_300,al_c,lg_1,q_85,enc_auto/011f31_f0edb3ba705e4712b539b563e0e4f63e~mv2.png) Cards Cloned/PAYPAL] WWW.Trusted-Best.bz Shop Dumps IST Pin/Card Clone SHOP, PRIVATE SNIFFER, BEST VALIDWe invite sellers,Welcome!, Emv Softwa09 janeiro 2025
Cards Cloned/PAYPAL] WWW.Trusted-Best.bz Shop Dumps IST Pin/Card Clone SHOP, PRIVATE SNIFFER, BEST VALIDWe invite sellers,Welcome!, Emv Softwa09 janeiro 2025 -
 ICQ Podcast Digital Group Hub — ICQ Amateur / Ham Radio Podcast09 janeiro 2025
ICQ Podcast Digital Group Hub — ICQ Amateur / Ham Radio Podcast09 janeiro 2025 -
 ICIQ Celebrates Science Week 2023 with exciting dissemination activities for all ages09 janeiro 2025
ICIQ Celebrates Science Week 2023 with exciting dissemination activities for all ages09 janeiro 2025
você pode gostar
-
 Usado: Jogo Need for Speed: ProStreet - Xbox 360 (Europeu) em09 janeiro 2025
Usado: Jogo Need for Speed: ProStreet - Xbox 360 (Europeu) em09 janeiro 2025 -
 Espada Dante Devil May Cry 4 Rebellion Em Aço - Tenda Medieval09 janeiro 2025
Espada Dante Devil May Cry 4 Rebellion Em Aço - Tenda Medieval09 janeiro 2025 -
 6 dicas para decorar uma árvore de Natal09 janeiro 2025
6 dicas para decorar uma árvore de Natal09 janeiro 2025 -
 Kratom Powder Green (Jetpackkratom)09 janeiro 2025
Kratom Powder Green (Jetpackkratom)09 janeiro 2025 -
 BUZZmod. Himura Kenshin Rurouni Kenshin Action Figure Limited Edition09 janeiro 2025
BUZZmod. Himura Kenshin Rurouni Kenshin Action Figure Limited Edition09 janeiro 2025 -
 Review: The Drifters' Girl at Manchester Opera House - The Mancunion09 janeiro 2025
Review: The Drifters' Girl at Manchester Opera House - The Mancunion09 janeiro 2025 -
 Hikity 9 Android 11 Car Stereo for Toyota RAV4 2001 2002 2003 2004 2005 2006 Wireless Carplay Android Auto GPS Navigation Stereo WiFi RDS Bluetoot Radio with 2GB Ram 32GB ROM : Electronics09 janeiro 2025
Hikity 9 Android 11 Car Stereo for Toyota RAV4 2001 2002 2003 2004 2005 2006 Wireless Carplay Android Auto GPS Navigation Stereo WiFi RDS Bluetoot Radio with 2GB Ram 32GB ROM : Electronics09 janeiro 2025 -
 Total War: Arena is shutting down early next year09 janeiro 2025
Total War: Arena is shutting down early next year09 janeiro 2025 -
 Doa Minum Air Zam Zam untuk Kesembuhan Penyakit dan Artinya09 janeiro 2025
Doa Minum Air Zam Zam untuk Kesembuhan Penyakit dan Artinya09 janeiro 2025 -
 Boneca Bebê Reborn Menina Realista Linda Toda Silicone 55 Cm09 janeiro 2025
Boneca Bebê Reborn Menina Realista Linda Toda Silicone 55 Cm09 janeiro 2025