A. Mean ASDAS and B. mean BASDAI to week 96. Safety set (N = 89).
Por um escritor misterioso
Last updated 27 fevereiro 2025
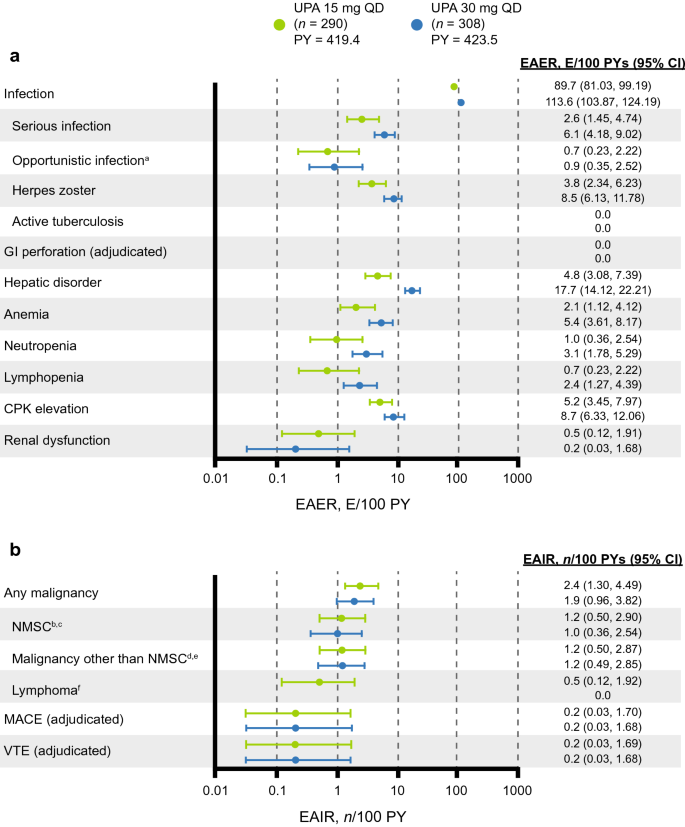
Upadacitinib in Patients with Psoriatic Arthritis and Inadequate Response to Biologics: 56-Week Data from the Randomized Controlled Phase 3 SELECT-PsA 2 Study

Monthly improvements in paid work productivity: ankylosing spondylitis
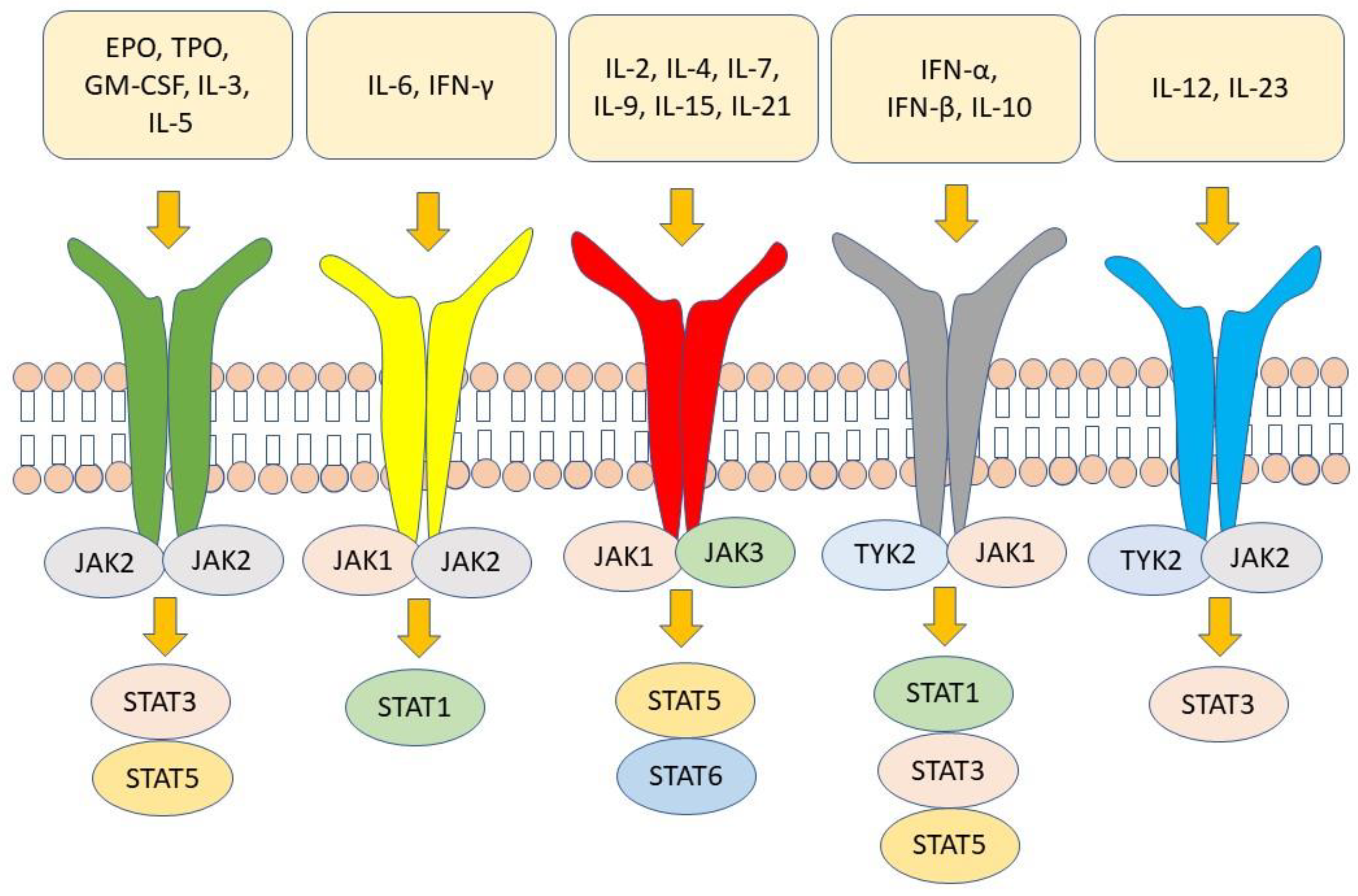
IJMS, Free Full-Text
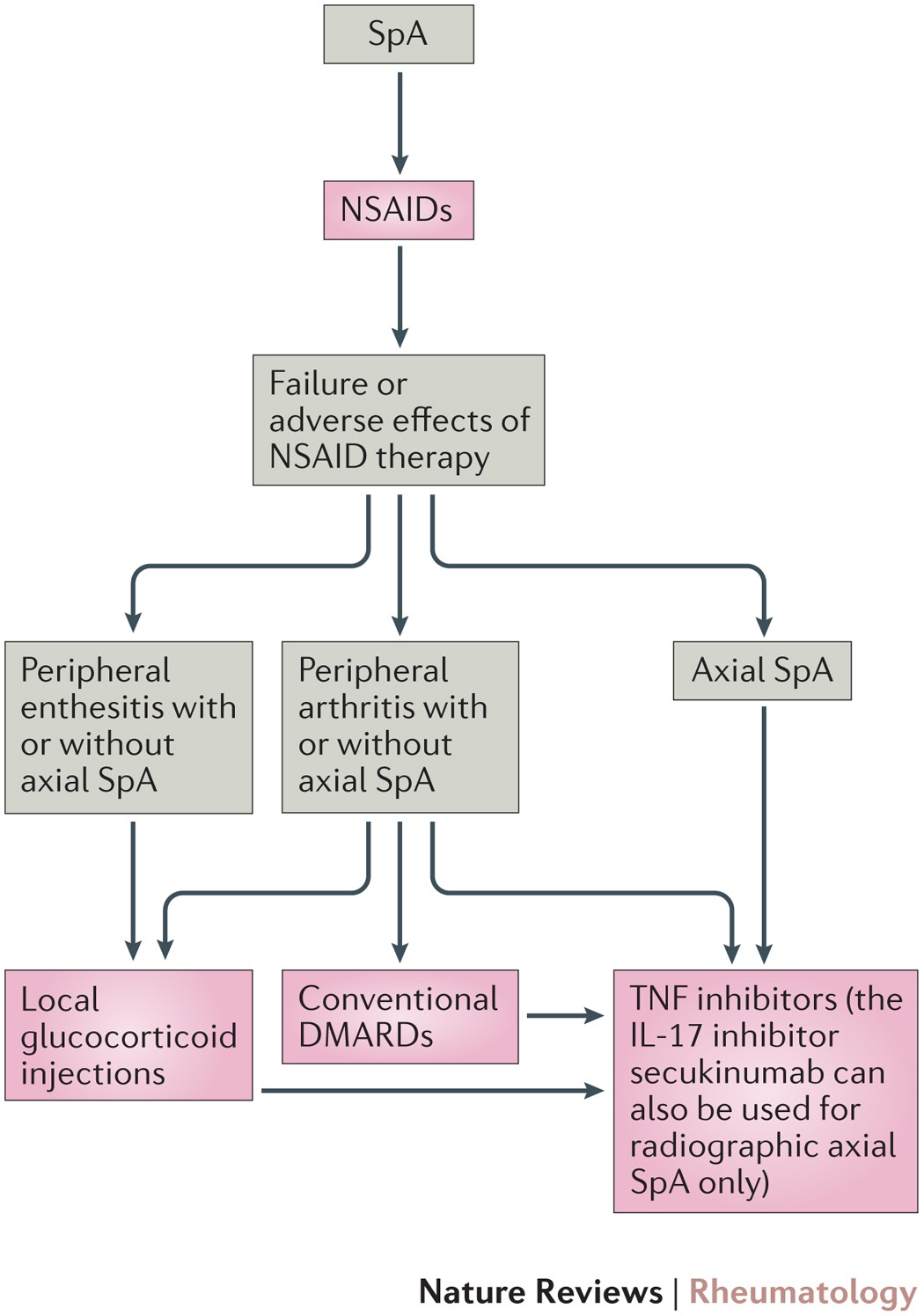
New evidence on the management of spondyloarthritis

PDF) The Minimum Clinically Important Improvement and Patient-acceptable Symptom State in the BASDAI and BASFI for Patients with Ankylosing Spondylitis
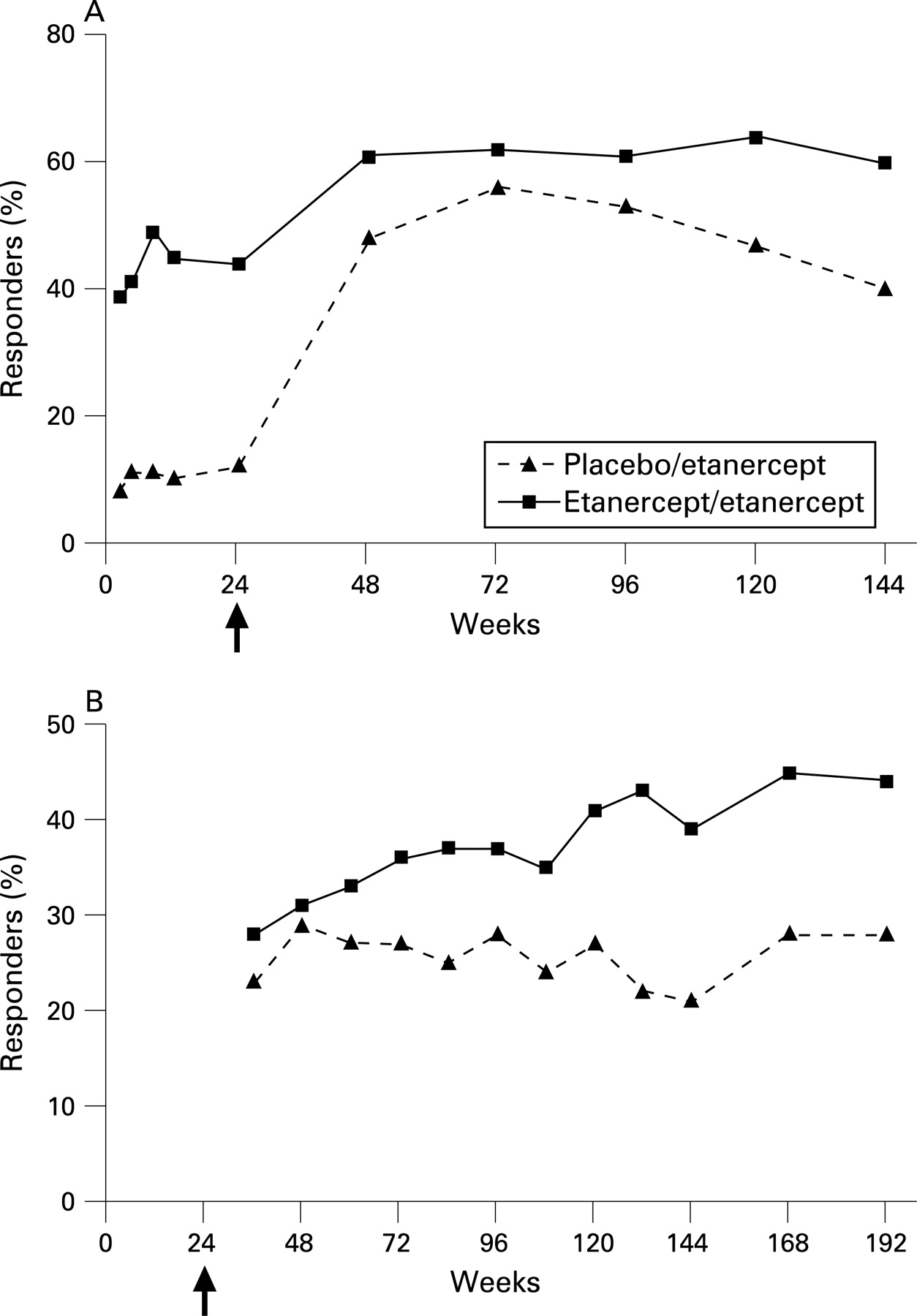
Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis

Oscar IRVIN-SELLERS, Medigene AG

WO2021067465A1 - Treating spondyloarthritic and psoriatic conditions with upadacitinib - Google Patents
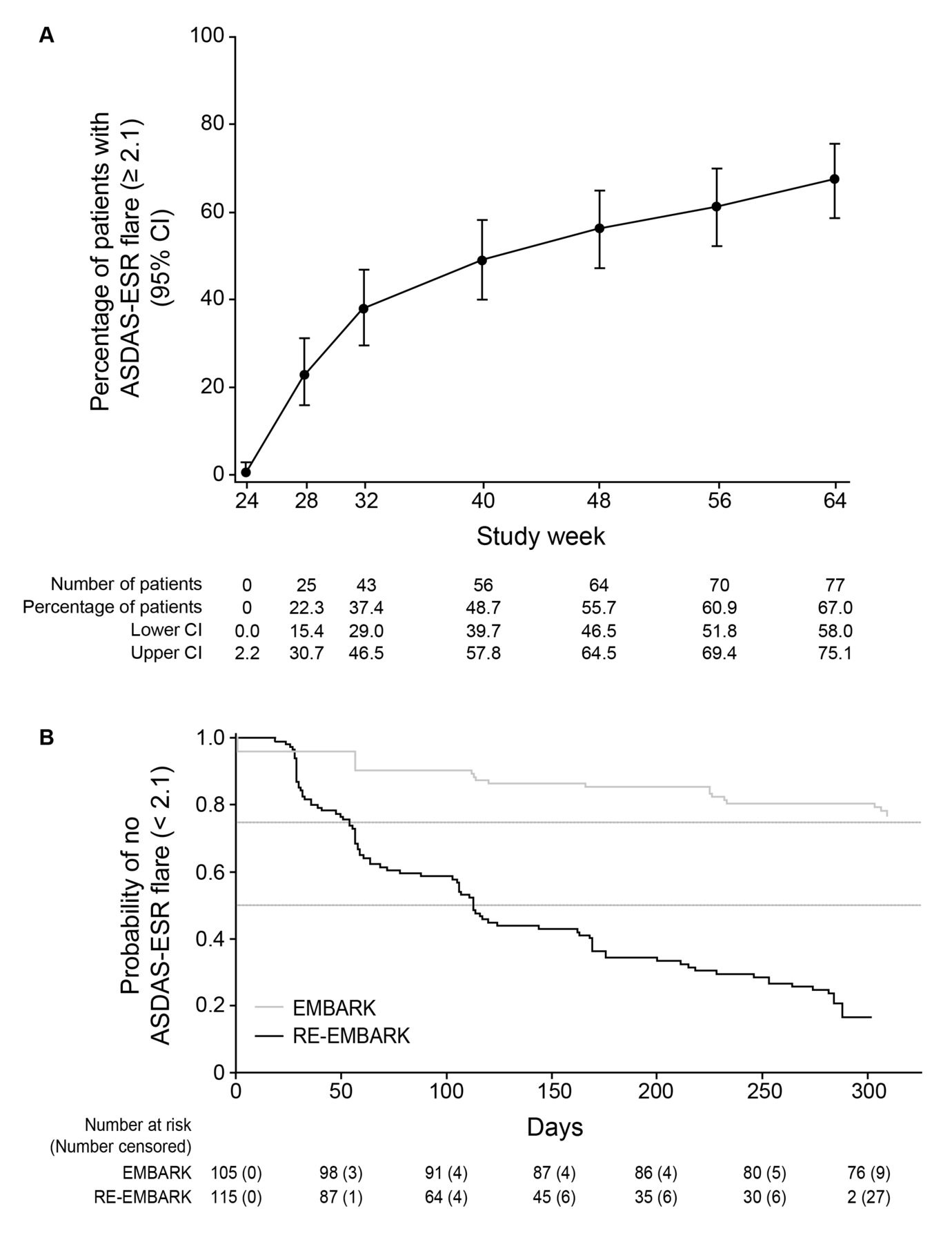
Etanercept Withdrawal and Retreatment in Nonradiographic Axial Spondyloarthritis: Results of RE-EMBARK, an Open-Label Phase IV Trial

Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial - The Lancet
Recomendado para você
-
 asdas dasdasd27 fevereiro 2025
asdas dasdasd27 fevereiro 2025 -
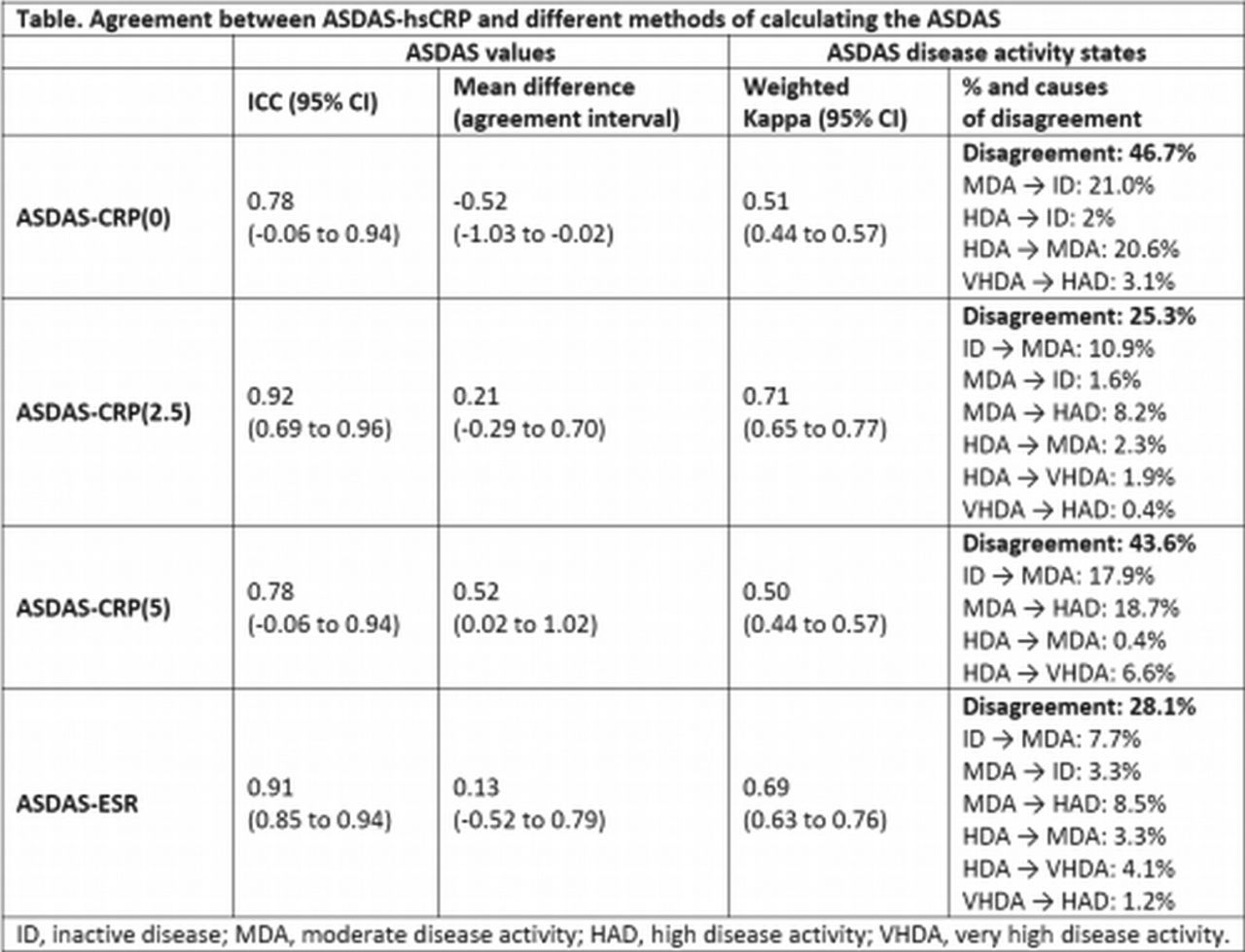 FRI0126 The Ankylosing Spondylitis Disease Activity Score (ASDAS): Defining the Best Calculation Method When the Conventional C-Reactive Protein (CRP) is below the Threshold of Detection - Results from the DESIR Cohort27 fevereiro 2025
FRI0126 The Ankylosing Spondylitis Disease Activity Score (ASDAS): Defining the Best Calculation Method When the Conventional C-Reactive Protein (CRP) is below the Threshold of Detection - Results from the DESIR Cohort27 fevereiro 2025 -
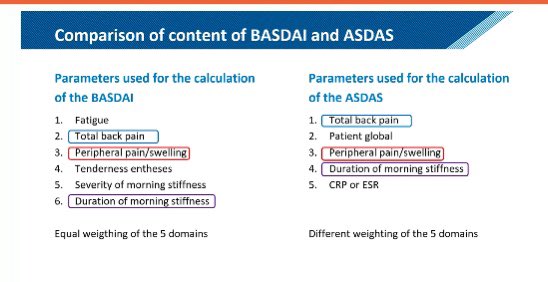 SMC Arthritis Forum/Dr.Hanady Manasfi on X: Comparison #BASDAI and #ASDAS #spondyloarthtopathy #EULAR2020 / X27 fevereiro 2025
SMC Arthritis Forum/Dr.Hanady Manasfi on X: Comparison #BASDAI and #ASDAS #spondyloarthtopathy #EULAR2020 / X27 fevereiro 2025 -
 Adobe InDesign icon with random file name asdas.indd | Greeting Card27 fevereiro 2025
Adobe InDesign icon with random file name asdas.indd | Greeting Card27 fevereiro 2025 -
 UK Grocery Retailer Deep Dive: ASDA27 fevereiro 2025
UK Grocery Retailer Deep Dive: ASDA27 fevereiro 2025 -
Asdas Svg Png Icon Free Download (#77015)27 fevereiro 2025
-
 Asdas Sticker - Asdas - Discover & Share GIFs27 fevereiro 2025
Asdas Sticker - Asdas - Discover & Share GIFs27 fevereiro 2025 -
 Asdas27 fevereiro 2025
Asdas27 fevereiro 2025 -
 Asda reveals real reason they use self-checkout cameras and it's not about data - Mirror Online27 fevereiro 2025
Asda reveals real reason they use self-checkout cameras and it's not about data - Mirror Online27 fevereiro 2025 -
 Adobe Audition CC icon with random file name asdas.sesx | Art Print27 fevereiro 2025
Adobe Audition CC icon with random file name asdas.sesx | Art Print27 fevereiro 2025
você pode gostar
-
 Hi Guys! So my MLBB account got hacked by an Indonesian and he is27 fevereiro 2025
Hi Guys! So my MLBB account got hacked by an Indonesian and he is27 fevereiro 2025 -
 VALORANT Champions 2023: Jogos da LOUD, Tabela, times, horários e resultados - Mais Esports27 fevereiro 2025
VALORANT Champions 2023: Jogos da LOUD, Tabela, times, horários e resultados - Mais Esports27 fevereiro 2025 -
 Memes: Their Messages & Variations Episode 2: Gru's Plan Memes27 fevereiro 2025
Memes: Their Messages & Variations Episode 2: Gru's Plan Memes27 fevereiro 2025 -
Mickey Mouse Clubhouse: Minnie's Bow-tique (2007 - DVD), 1 Count - King Soopers27 fevereiro 2025
-
 CoopDojo Opinion: Destiny's Skolas is the best FPS boss fight ever – Coop Dojo27 fevereiro 2025
CoopDojo Opinion: Destiny's Skolas is the best FPS boss fight ever – Coop Dojo27 fevereiro 2025 -
Furar a fila na Cacheta Zingplay!!!27 fevereiro 2025
-
 Sonic Speed Simulator Codes Roblox27 fevereiro 2025
Sonic Speed Simulator Codes Roblox27 fevereiro 2025 -
 RADIONICA „GRAFFITI SU UMJETNOST, NE VANDALIZAM“ - Blog - Obrtnička škola Požega27 fevereiro 2025
RADIONICA „GRAFFITI SU UMJETNOST, NE VANDALIZAM“ - Blog - Obrtnička škola Požega27 fevereiro 2025 -
 Cat Warrior Puzzle ✔️ Art Puzzles ✔️ Puzzles Print27 fevereiro 2025
Cat Warrior Puzzle ✔️ Art Puzzles ✔️ Puzzles Print27 fevereiro 2025 -
 bolo free fire pasta americana #bolofreefire #jogofreefire #freefire27 fevereiro 2025
bolo free fire pasta americana #bolofreefire #jogofreefire #freefire27 fevereiro 2025

